Investigating the Potential Signaling Pathways That Regulate Activation of the Novel PKC Downstream of Serotonin in Aplysia
- PMID: 28002451
- PMCID: PMC5176290
- DOI: 10.1371/journal.pone.0168411
Investigating the Potential Signaling Pathways That Regulate Activation of the Novel PKC Downstream of Serotonin in Aplysia
Abstract
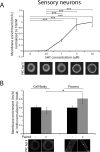
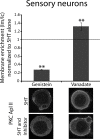
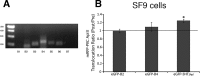
Activation of the novel PKC Apl II in sensory neurons by serotonin (5HT) underlies the ability of 5HT to reverse synaptic depression, but the pathway from 5HT to PKC Apl II activation remains unclear. Here we find no evidence for the Aplysia-specific B receptors, or for adenylate cyclase activation, to translocate fluorescently-tagged PKC Apl II. Using an anti-PKC Apl II antibody, we monitor translocation of endogenous PKC Apl II and determine the dose response for PKC Apl II translocation, both in isolated sensory neurons and sensory neurons coupled with motor neurons. Using this assay, we confirm an important role for tyrosine kinase activation in 5HT mediated PKC Apl II translocation, but rule out roles for intracellular tyrosine kinases, epidermal growth factor (EGF) receptors and Trk kinases in this response. A partial inhibition of translocation by a fibroblast growth factor (FGF)-receptor inhibitor led us to clone the Aplysia FGF receptor. Since a number of related receptors have been recently characterized, we use bioinformatics to define the relationship between these receptors and find a single FGF receptor orthologue in Aplysia. However, expression of the FGF receptor did not affect translocation or allow it in motor neurons where 5HT does not normally cause PKC Apl II translocation. These results suggest that additional receptor tyrosine kinases (RTKs) or other molecules must also be involved in translocation of PKC Apl II.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Regulation of protein kinase C Apl II by serotonin receptors in Aplysia.J Neurochem. 2010 Nov;115(4):994-1006. doi: 10.1111/j.1471-4159.2010.06986.x. Epub 2010 Oct 5. J Neurochem. 2010. PMID: 20964689
-
Ca2+-independent protein kinase C Apl II mediates the serotonin-induced facilitation at depressed aplysia sensorimotor synapses.J Neurosci. 2001 Feb 15;21(4):1247-56. doi: 10.1523/JNEUROSCI.21-04-01247.2001. J Neurosci. 2001. PMID: 11160395 Free PMC article.
-
Isoform specificity of PKC translocation in living Aplysia sensory neurons and a role for Ca2+-dependent PKC APL I in the induction of intermediate-term facilitation.J Neurosci. 2006 Aug 23;26(34):8847-56. doi: 10.1523/JNEUROSCI.1919-06.2006. J Neurosci. 2006. PMID: 16928874 Free PMC article.
-
Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes.Learn Mem. 2003 Sep-Oct;10(5):373-86. doi: 10.1101/lm.66103. Learn Mem. 2003. PMID: 14557610 Free PMC article. Review.
-
Isoform specificity of protein kinase Cs in synaptic plasticity.Learn Mem. 2007 Apr 2;14(4):236-46. doi: 10.1101/lm.469707. Print 2007 Apr. Learn Mem. 2007. PMID: 17404386 Review.
Cited by
-
Low-intensity exercise prevents cardiac inflammation through the NF-κB/TNFα pathway in insulin-resistant male rats.Mol Cell Biochem. 2025 Aug;480(8):4757-4767. doi: 10.1007/s11010-025-05288-x. Epub 2025 Apr 17. Mol Cell Biochem. 2025. PMID: 40244304
-
Transcriptional correlates of memory maintenance following long-term sensitization of Aplysia californica.Learn Mem. 2017 Sep 15;24(10):502-515. doi: 10.1101/lm.045450.117. Print 2017 Oct. Learn Mem. 2017. PMID: 28916625 Free PMC article.
References
-
- Castellucci V, Pinsker H, Kupfermann I, Kandel ER (1970) Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167: 1745–1748. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

