Dose-Finding Study of Omeprazole on Gastric pH in Neonates with Gastro-Esophageal Acid Reflux Using a Bayesian Sequential Approach
- PMID: 28002471
- PMCID: PMC5176365
- DOI: 10.1371/journal.pone.0166207
Dose-Finding Study of Omeprazole on Gastric pH in Neonates with Gastro-Esophageal Acid Reflux Using a Bayesian Sequential Approach
Abstract
Objective: Proton pump inhibitors are frequently administered on clinical symptoms in neonates but benefit remains controversial. Clinical trials validating omeprazole dosage in neonates are limited. The objective of this trial was to determine the minimum effective dose (MED) of omeprazole to treat pathological acid reflux in neonates using reflux index as surrogate marker.
Design: Double blind dose-finding trial with continual reassessment method of individual dose administration using a Bayesian approach, aiming to select drug dose as close as possible to the predefined target level of efficacy (with a credibility interval of 95%).
Setting: Neonatal Intensive Care unit of the Robert Debré University Hospital in Paris, France.
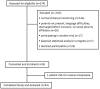
Patients: Neonates with a postmenstrual age ≥ 35 weeks and a pathologic 24-hour intra-esophageal pH monitoring defined by a reflux index ≥ 5% over 24 hours were considered for participation. Recruitment was stratified to 3 groups according to gestational age at birth.
Intervention: Five preselected doses of oral omeprazole from 1 to 3 mg/kg/day.
Main outcome measures: Primary outcome, measured at 35 weeks postmenstrual age or more, was a reflux index <5% during the 24-h pH monitoring registered 72±24 hours after omeprazole initiation.
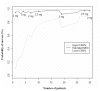
Results: Fifty-four neonates with a reflux index ranging from 5.06 to 27.7% were included. Median age was 37.5 days and median postmenstrual age was 36 weeks. In neonates born at less than 32 weeks of GA (n = 30), the MED was 2.5mg/kg/day with an estimated mean posterior probability of success of 97.7% (95% credibility interval: 90.3-99.7%). The MED was 1mg/kg/day for neonates born at more than 32 GA (n = 24).
Conclusions: Omeprazole is extensively prescribed on clinical symptoms but efficacy is not demonstrated while safety concerns do exist. When treatment is required, the daily dose needs to be validated in preterm and term neonates. Optimal doses of omeprazole to increase gastric pH and decrease reflux index below 5% over 24 hours, determined using an adaptive Bayesian design differ among neonates. Both gestational and postnatal ages account for these differences but their differential impact on omeprazole doses remains to be determined.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Boyle JT. Acid secretion from birth to adulthood. J Pediatr Gastroenterol Nutr. 2003;37 Suppl 1:S12–6. Epub 2003/12/20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

