Chemoenzymatic Approach for the Preparation of Asymmetric Bi-, Tri-, and Tetra-Antennary N-Glycans from a Common Precursor
- PMID: 28002670
- PMCID: PMC5461401
- DOI: 10.1021/jacs.6b12080
Chemoenzymatic Approach for the Preparation of Asymmetric Bi-, Tri-, and Tetra-Antennary N-Glycans from a Common Precursor
Abstract
Progress in glycoscience is hampered by a lack of well-defined complex oligosaccharide standards that are needed to fabricate the next generation of microarrays, to develop analytical protocols to determine exact structures of isolated glycans, and to elucidate pathways of glycan biosynthesis. We describe here a chemoenzymatic methodology that makes it possible, for the first time, to prepare any bi-, tri-, and tetra-antennary asymmetric N-glycan from a single precursor. It is based on the chemical synthesis of a tetra-antennary glycan that has N-acetylglucosamine (GlcNAc), N-acetyllactosamine (LacNAc), and unnatural Galα(1,4)-GlcNAc and Manβ(1,4)-GlcNAc appendages. Mammalian glycosyltransferases recognize only the terminal LacNAc moiety as a substrate, and thus this structure can be uniquely extended. Next, the β-GlcNAc terminating antenna can be converted into LacNAc by galactosylation and can then be enzymatically modified into a complex structure. The unnatural α-Gal and β-Man terminating antennae can sequentially be decaged by an appropriate glycosidase to liberate a terminal β-GlcNAc moiety, which can be converted into LacNAc and then elaborated by a panel of glycosyltransferases. Asymmetric bi- and triantennary glycans could be obtained by removal of a terminal β-GlcNAc moiety by treatment with β-N-acetylglucosaminidase and selective extension of the other arms. The power of the methodology is demonstrated by the preparation of an asymmetric tetra-antennary N-glycan found in human breast carcinoma tissue, which represents the most complex N-glycan ever synthesized. Multistage mass spectrometry of the two isomeric triantennary glycans uncovered unique fragment ions that will facilitate identification of exact structures of glycans in biological samples.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
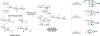




Similar articles
-
Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation.J Biol Chem. 2011 Jun 17;286(24):21717-31. doi: 10.1074/jbc.M110.194597. Epub 2011 Apr 14. J Biol Chem. 2011. PMID: 21493714 Free PMC article.
-
Asymmetrical Bi-antennary Glycans Prepared by a Stop-and-Go Strategy Reveal Receptor Binding Evolution of Human Influenza A Viruses.bioRxiv [Preprint]. 2023 Nov 9:2023.11.08.566285. doi: 10.1101/2023.11.08.566285. bioRxiv. 2023. Update in: JACS Au. 2024 Jan 23;4(2):607-618. doi: 10.1021/jacsau.3c00695. PMID: 37986780 Free PMC article. Updated. Preprint.
-
Chemoenzymatic Synthesis of Tri-antennary N-Glycans Terminating in Sialyl-Lewisx Reveals the Importance of Glycan Complexity for Influenza A Virus Receptor Binding.Chemistry. 2024 Jun 6;30(32):e202401108. doi: 10.1002/chem.202401108. Epub 2024 May 8. Chemistry. 2024. PMID: 38567703 Free PMC article.
-
Recent progress in chemoenzymatic synthesis of human glycans.Org Biomol Chem. 2024 Oct 2;22(38):7767-7785. doi: 10.1039/d4ob01006j. Org Biomol Chem. 2024. PMID: 39246045 Review.
-
The role of the GlcNAc(beta)1,2Man(alpha)- moiety in mammalian development. Null mutations of the genes encoding UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I and UDP-N-acetylglucosamine:alpha-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I.2 cause embryonic lethality and congenital muscular dystrophy in mice and men, respectively.Biochim Biophys Acta. 2002 Dec 19;1573(3):292-300. doi: 10.1016/s0304-4165(02)00396-3. Biochim Biophys Acta. 2002. PMID: 12417411 Review.
Cited by
-
Divergent Enzymatic Assembly of a Comprehensive 64-Membered IgG N-Glycan Library for Functional Glycomics.Adv Sci (Weinh). 2023 Oct;10(30):e2303832. doi: 10.1002/advs.202303832. Epub 2023 Aug 26. Adv Sci (Weinh). 2023. PMID: 37632720 Free PMC article.
-
Chemoenzymatic synthesis of glycopeptides bearing rare N-glycan sequences with or without bisecting GlcNAc.Chem Sci. 2018 Aug 31;9(43):8194-8206. doi: 10.1039/c8sc02457j. eCollection 2018 Nov 21. Chem Sci. 2018. PMID: 30542567 Free PMC article.
-
Decoding glycan protein interactions by a new class of asymmetric N-glycans.Org Biomol Chem. 2017 Oct 31;15(42):8946-8951. doi: 10.1039/c7ob02303k. Org Biomol Chem. 2017. PMID: 29043371 Free PMC article.
-
A Biomimetic Synthetic Strategy Can Provide Keratan Sulfate I and II Oligosaccharides with Diverse Fucosylation and Sulfation Patterns.J Am Chem Soc. 2024 Apr 3;146(13):9230-9240. doi: 10.1021/jacs.4c00363. Epub 2024 Mar 17. J Am Chem Soc. 2024. PMID: 38494637 Free PMC article.
-
Glycoproteomics-Compatible MS/MS-Based Quantification of Glycopeptide Isomers.Anal Chem. 2023 Jun 27;95(25):9605-9614. doi: 10.1021/acs.analchem.3c01319. Epub 2023 Jun 15. Anal Chem. 2023. PMID: 37319314 Free PMC article.
References
-
- Spik G, Debruyne V, Montreuil J, van Halbeek H, Vliegenthart JFG. FEBS Lett. 1985;183:65–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

