Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model
- PMID: 28002796
- PMCID: PMC5354867
- DOI: 10.18632/oncotarget.13972
Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model
Abstract
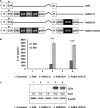
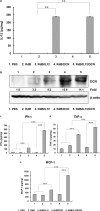
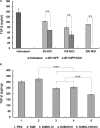
Interleukin (IL)-12 is a potent antitumor cytokine. However, immunosuppressive tumor microenvironments containing transforming growth factor-β (TGF-β) attenuate cytokine-mediated antitumor immune responses. To enhance the efficacy of IL-12-mediated cancer immunotherapy, decorin (DCN) was explored as an adjuvant for overcoming TGF-β-mediated immunosuppression. We designed and generated a novel oncolytic adenovirus (Ad) coexpressing IL-12 and DCN (RdB/IL12/DCN). RdB/IL12/DCN-treated tumors showed significantly greater levels of interferon (IFN)-γ, tumor necrosis factor-α, monocyte chemoattractant protein-1, and IFN-γ-secreting immune cells than tumors treated with cognate control oncolytic Ad expressing a single therapeutic gene (RdB/DCN or RdB/IL12). Moreover, RdB/IL12/DCN attenuated intratumoral TGF-β expression, which positively correlated with reduction of Treg cells in draining lymph nodes and tumor tissues. Furthermore, tumor tissue treated with RdB/IL12/DCN showed increases infiltration of CD8+ T cells and proficient viral spreading within tumor tissues. These results demonstrated that an oncolytic Ad co-expressing IL-12 and DCN induces a potent antitumor immune response via restoration of antitumor immune function in a weakly immunogenic murine 4T1 orthotopic breast cancer model. These findings provide new insights into the therapeutic mechanisms of IL-12 plus DCN, making it a promising cancer immunotherapeutic agent for overcoming tumor-induced immunosuppression.
Keywords: IL-12; TGF-β; Treg; decorin; oncolytic adenovirus.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




References
-
- Kruger C, Greten TF, Korangy F. Immune based therapies in cancer. Histol Histopathol. 2007;22:687–696. - PubMed
-
- Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M. Elimination of CD4(+) T cells enhances anti-tumor effect of locally secreted interleukin-12 on B16 mouse melanoma and induces vitiligo-like coat color alteration. J Invest Dermatol. 2000;115:1059–1064. - PubMed
-
- Bubenik J. Genetically modified tumour vaccines carrying inserted genes for immunoregulatory molecules. Folia Biol (Praha) 1996;42:295–304. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

