The drugs that mostly frequently induce acute kidney injury: a case - noncase study of a pharmacovigilance database
- PMID: 28002877
- PMCID: PMC5427222
- DOI: 10.1111/bcp.13216
The drugs that mostly frequently induce acute kidney injury: a case - noncase study of a pharmacovigilance database
Abstract
Aims: Acute kidney injury (AKI) is associated with a high hospitalization rate, accelerated long-term decline in kidney function and a high mortality rate. Adverse drug reactions (ADRs) constitute one of the most important modifiable factors in the context of AKI. Most studies of drug-induced AKI have focused on a sole drug class. The objective of the present study was to establish a comprehensive overview of drug-induced AKI on the basis of spontaneously reported ADRs in the French national pharmacovigilance database (FPVD).
Methods: We performed a case-noncase study of drug-induced AKI. Cases corresponded to the reports of AKI recorded in the FPVD between 1 January 2015 and 31 December 2015. The noncases corresponded to all other spontaneously reported ADRs (excluding AKI) recorded in the FPVD during the same period. Data were expressed as the reporting odds ratio (ROR) and the 95% confidence interval.
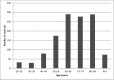
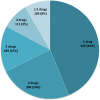
Results: Of the 38 782 ADRs recorded in the FPVD during the study period, 3.2% were classified as cases of AKI. A total of 1254 patients experienced AKI (males: 55%; mean age ± standard deviation: 68.7 ± 15.0 years). Overall, 15.2% of the patients required renal replacement therapy. Two or more concomitantly administered drugs were involved in 66% of the cases of AKI. The most frequently implicated drug classes were antibacterial agents for systemic use (29.5%), diuretics (18.5%), agents acting on the renin-angiotensin system (16.3%), antineoplastic agents (10.2%) and anti-inflammatory agents (5.4%). Gentamicin, eplerenone, spironolactone, candesartan, cisplatin and acyclovir had the highest RORs (>10).
Conclusion: A comprehensive study of a national pharmacovigilance database enabled us to identify the drug classes that most frequently induced AKI. Even though most of the identified drugs were already known to induce AKI, the present work should raise physicians' awareness of the compounds responsible for triggering this potentially life-threatening condition.
Keywords: acute kidney injury; drug; nephrotoxicity; pharmacovigilance database.
© 2016 The British Pharmacological Society.
Figures

References
-
- Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66: 1613–1621. - PubMed
-
- Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population‐based study. J Am Soc Nephrol 2007; 18: 1292–1298. - PubMed
-
- Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI‐EPI study. Intensive Care Med 2015; 41: 1411–1423. - PubMed
-
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 2006; 17: 1135–1142. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

