Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis
- PMID: 28003097
- PMCID: PMC5285449
- DOI: 10.1053/j.gastro.2016.10.047
Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis
Abstract
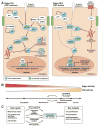
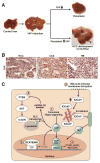
The Hippo signaling pathway, also known as the Salvador-Warts-Hippo pathway, is a regulator of organ size. The pathway takes its name from the Drosophila protein kinase, Hippo (STK4/MST1 and STK3/MST2 in mammals), which, when inactivated, leads to considerable tissue overgrowth. In mammals, MST1 and MST2 negatively regulate the transcriptional co-activators yes-associated protein 1 and WW domain containing transcription regulator 1 (WWTR1/TAZ), which together regulate expression of genes that control proliferation, survival, and differentiation. Yes-associated protein 1 and TAZ activation have been associated with liver development, regeneration, and tumorigenesis. How their activity is dynamically regulated in these contexts is just beginning to be elucidated. We review the mechanisms of Hippo signaling in the liver and explore outstanding questions for future research.
Keywords: TAZ; WWTR1; YAP1; YES-Associated Protein.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. - PubMed
-
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. - PubMed
-
- Dutkowski P, Linecker M, DeOliveira ML, et al. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307–23. - PubMed
-
- Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc. 2011;43:884–7. - PubMed
-
- Tanaka A, Tanaka K, Tokuka A, et al. Graft size-matching in living related partial liver transplantation in relation to tissue oxygenation and metabolic capacity. Transpl Int. 1996;9:15–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

