Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells
- PMID: 28003188
- PMCID: PMC5336590
- DOI: 10.1152/ajprenal.00271.2016
Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells
Abstract
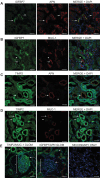
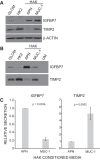
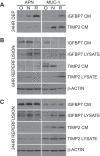
We have characterized the expression and secretion of the acute kidney injury (AKI) biomarkers insulin-like growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) in human kidney epithelial cells in primary cell culture and tissue. We established cell culture model systems of primary kidney cells of proximal and distal tubule origin and observed that both proteins are indeed expressed and secreted in both tubule cell types in vitro. However, TIMP-2 is both expressed and secreted preferentially by cells of distal tubule origin, while IGFBP7 is equally expressed across tubule cell types yet preferentially secreted by cells of proximal tubule origin. In human kidney tissue, strong staining of IGFBP7 was seen in the luminal brush-border region of a subset of proximal tubule cells, and TIMP-2 stained intracellularly in distal tubules. Additionally, while some tubular colocalization of both biomarkers was identified with the injury markers kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin, both biomarkers could also be seen alone, suggesting the possibility for differential mechanistic and/or temporal profiles of regulation of these early AKI biomarkers from known markers of injury. Last, an in vitro model of ischemia-reperfusion demonstrated enhancement of secretion of both markers early after reperfusion. This work provides a rationale for further investigation of these markers for their potential role in the pathogenesis of acute kidney injury.
Keywords: IGFBP7; acute kidney injury; and TIMP-2; biomarkers.
Copyright © 2017 the American Physiological Society.
Figures




Similar articles
-
Mechanisms Underlying Increased TIMP2 and IGFBP7 Urinary Excretion in Experimental AKI.J Am Soc Nephrol. 2018 Aug;29(8):2157-2167. doi: 10.1681/ASN.2018030265. Epub 2018 Jul 6. J Am Soc Nephrol. 2018. PMID: 29980651 Free PMC article.
-
Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice.Clin Chem Lab Med. 2019 Apr 24;57(5):567-576. doi: 10.1515/cclm-2018-0776. Clin Chem Lab Med. 2019. PMID: 30179848 Review.
-
Urinary beta-2 microglobulin increases whereas TIMP-2 and IGFBP7 decline after unilateral nephrectomy in healthy kidney donors.Sci Rep. 2024 Jun 5;14(1):12901. doi: 10.1038/s41598-024-62246-1. Sci Rep. 2024. PMID: 38839764 Free PMC article.
-
Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury.Dis Markers. 2015;2015:158658. doi: 10.1155/2015/158658. Epub 2015 Mar 18. Dis Markers. 2015. PMID: 25866432 Free PMC article.
-
Biomarkers for acute kidney injury in children - where are we now?Curr Opin Pediatr. 2023 Apr 1;35(2):245-250. doi: 10.1097/MOP.0000000000001217. Epub 2023 Jan 5. Curr Opin Pediatr. 2023. PMID: 36601976 Review.
Cited by
-
Kidney Cell Cycle Arrest and Cardiac Biomarkers and Acute Kidney Injury Following Angiography: The Prevention of Serious Adverse Events Following Angiography (PRESERVE) Study.Kidney Med. 2022 Dec 21;5(3):100592. doi: 10.1016/j.xkme.2022.100592. eCollection 2023 Mar. Kidney Med. 2022. PMID: 36874509 Free PMC article.
-
Urinary Tissue Inhibitor of Metalloproteinases-2 and Insulin-Like Growth Factor-Binding Protein 7 Do Not Correlate With Disease Severity in ADPKD Patients.Kidney Int Rep. 2019 Mar 22;4(6):833-841. doi: 10.1016/j.ekir.2019.03.011. eCollection 2019 Jun. Kidney Int Rep. 2019. PMID: 31194166 Free PMC article.
-
Kidney injury risk during prolonged exposure to current and projected wet bulb temperatures occurring during extreme heat events in healthy young men.J Appl Physiol (1985). 2022 Jul 1;133(1):27-40. doi: 10.1152/japplphysiol.00601.2021. Epub 2022 May 26. J Appl Physiol (1985). 2022. PMID: 35616302 Free PMC article.
-
Time-dependent effects of histone deacetylase inhibition in sepsis-associated acute kidney injury.Intensive Care Med Exp. 2020 Feb 7;8(1):9. doi: 10.1186/s40635-020-0297-3. Intensive Care Med Exp. 2020. PMID: 32034542 Free PMC article.
-
Issues of Acute Kidney Injury Staging and Management in Sepsis and Critical Illness: A Narrative Review.Int J Mol Sci. 2017 Jun 28;18(7):1387. doi: 10.3390/ijms18071387. Int J Mol Sci. 2017. PMID: 28657585 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

