Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2
- PMID: 28003219
- PMCID: PMC5336520
- DOI: 10.1161/CIRCRESAHA.116.309833
Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2
Abstract
Rationale: Direct conversion or reprogramming of human postnatal cells into endothelial cells (ECs), bypassing stem or progenitor cell status, is crucial for regenerative medicine, cell therapy, and pathophysiological investigation but has remained largely unexplored.
Objective: We sought to directly reprogram human postnatal dermal fibroblasts to ECs with vasculogenic and endothelial transcription factors and determine their vascularizing and therapeutic potential.
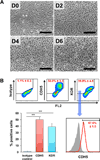
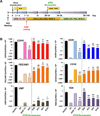
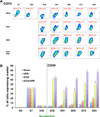
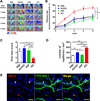
Methods and results: We utilized various combinations of 7 EC transcription factors to transduce human postnatal dermal fibroblasts and found that ER71/ETV2 (ETS variant 2) alone best induced endothelial features. KDR+ (kinase insert domain receptor) cells sorted at day 7 from ER71/ETV2-transduced human postnatal dermal fibroblasts showed less mature but enriched endothelial characteristics and thus were referred to as early reprogrammed ECs (rECs), and did not undergo maturation by further culture. After a period of several weeks' transgene-free culture followed by transient reinduction of ER71/ETV2, early rECs matured during 3 months of culture and showed reduced ETV2 expression, reaching a mature phenotype similar to postnatal human ECs. These were termed late rECs. While early rECs exhibited an immature phenotype, their implantation into ischemic hindlimbs induced enhanced recovery from ischemia. These 2 rECs showed clear capacity for contributing to new vessel formation through direct vascular incorporation in vivo. Paracrine or proangiogenic effects of implanted early rECs played a significant role in repairing hindlimb ischemia.
Conclusions: This study for the first time demonstrates that ER71/ETV2 alone can directly reprogram human postnatal cells to functional, mature ECs after an intervening transgene-free period. These rECs could be valuable for cell therapy, personalized disease investigation, and exploration of the reprogramming process.
Keywords: cell therapy; endothelial cells; fibroblasts; ischemia; regenerative medicine.
© 2016 American Heart Association, Inc.
Figures









Comment in
-
Reprogrammed Human Endothelial Cells: A Novel Cell Source for Regenerative Vascular Medicine.Circ Res. 2017 Mar 3;120(5):756-758. doi: 10.1161/CIRCRESAHA.117.310573. Circ Res. 2017. PMID: 28254795 Free PMC article. No abstract available.
References
-
- O'Neill TJt, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. - PubMed
-
- Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. - PubMed
-
- Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–252. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

