Heterogeneity of neuroanatomical patterns in prodromal Alzheimer's disease: links to cognition, progression and biomarkers
- PMID: 28003242
- PMCID: PMC5837514
- DOI: 10.1093/brain/aww319
Heterogeneity of neuroanatomical patterns in prodromal Alzheimer's disease: links to cognition, progression and biomarkers
Abstract
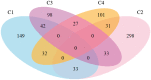
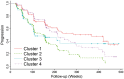
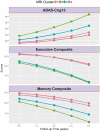
See Coulthard and Knight (doi:10.1093/aww335) for a scientific commentary on this article.Individuals with mild cognitive impairment and Alzheimer's disease clinical diagnoses can display significant phenotypic heterogeneity. This variability likely reflects underlying genetic, environmental and neuropathological differences. Characterizing this heterogeneity is important for precision diagnostics, personalized predictions, and recruitment of relatively homogeneous sets of patients into clinical trials. In this study, we apply state-of-the-art semi-supervised machine learning methods to the Alzheimer's disease Neuroimaging cohort (ADNI) to elucidate the heterogeneity of neuroanatomical differences between subjects with mild cognitive impairment (n = 530) and Alzheimer's disease (n = 314) and cognitively normal individuals (n = 399), thereby adding to an increasing literature aiming to establish neuroanatomical and neuropathological (e.g. amyloid and tau deposition) dimensions in Alzheimer's disease and its prodromal stages. These dimensional approaches aim to provide surrogate measures of heterogeneous underlying pathologic processes leading to cognitive impairment. We relate these neuroimaging patterns to cerebrospinal fluid biomarkers, white matter hyperintensities, cognitive and clinical measures, and longitudinal trajectories. We identified four such atrophy patterns: (i) individuals with largely normal neuroanatomical profiles, who also turned out to have the least abnormal cognitive and cerebrospinal fluid biomarker profiles and the slowest clinical progression during follow-up; (ii) individuals with classical Alzheimer's disease neuroanatomical, cognitive, cerebrospinal fluid biomarkers and clinical profile, who presented the fastest clinical progression; (iii) individuals with a diffuse pattern of atrophy with relatively less pronounced involvement of the medial temporal lobe, abnormal cerebrospinal fluid amyloid-β1-42 values, and proportionally greater executive impairment; and (iv) individuals with notably focal involvement of the medial temporal lobe and a slow steady progression, likely representing in early Alzheimer's disease stages. These four atrophy patterns effectively define a 4-dimensional categorization of neuroanatomical alterations in mild cognitive impairment and Alzheimer's disease that can complement existing dimensional approaches for staging Alzheimer's disease using a variety of biomarkers, which offer the potential for enabling precision diagnostics and prognostics, as well as targeted patient recruitment of relatively homogeneous subgroups of subjects for clinical trials.
Keywords: dementia; magnetic resonance imaging; mild cognitive impairment; neuroanatomical heterogeneity; pattern analysis.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Refining Alzheimer's disease diagnosis with MRI.Brain. 2017 Mar 1;140(3):524-526. doi: 10.1093/brain/aww335. Brain. 2017. PMID: 28364551 No abstract available.
References
-
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K. et al. Focal cortical presentations of Alzheimer’s disease. Brain 2007; 130: 2636–45. - PubMed
-
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage 2000; 11: 805–21. - PubMed
-
- Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer’s disease. Expert Rev Neurother 2008; 8: 1691–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

