KCTD Hetero-oligomers Confer Unique Kinetic Properties on Hippocampal GABAB Receptor-Induced K+ Currents
- PMID: 28003345
- PMCID: PMC6596860
- DOI: 10.1523/JNEUROSCI.2181-16.2016
KCTD Hetero-oligomers Confer Unique Kinetic Properties on Hippocampal GABAB Receptor-Induced K+ Currents
Abstract
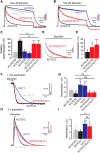
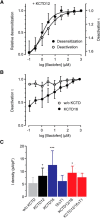
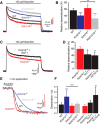
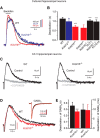
GABAB receptors are the G-protein coupled receptors for the main inhibitory neurotransmitter in the brain, GABA. GABAB receptors were shown to associate with homo-oligomers of auxiliary KCTD8, KCTD12, KCTD12b, and KCTD16 subunits (named after their T1 K+-channel tetramerization domain) that regulate G-protein signaling of the receptor. Here we provide evidence that GABAB receptors also associate with hetero-oligomers of KCTD subunits. Coimmunoprecipitation experiments indicate that two-thirds of the KCTD16 proteins in the hippocampus of adult mice associate with KCTD12. We show that the KCTD proteins hetero-oligomerize through self-interacting T1 and H1 homology domains. Bioluminescence resonance energy transfer measurements in live cells reveal that KCTD12/KCTD16 hetero-oligomers associate with both the receptor and the G-protein. Electrophysiological experiments demonstrate that KCTD12/KCTD16 hetero-oligomers impart unique kinetic properties on G-protein-activated Kir3 currents. During prolonged receptor activation (one min) KCTD12/KCTD16 hetero-oligomers produce moderately desensitizing fast deactivating K+ currents, whereas KCTD12 and KCTD16 homo-oligomers produce strongly desensitizing fast deactivating currents and nondesensitizing slowly deactivating currents, respectively. During short activation (2 s) KCTD12/KCTD16 hetero-oligomers produce nondesensitizing slowly deactivating currents. Electrophysiological recordings from hippocampal neurons of KCTD knock-out mice are consistent with these findings and indicate that KCTD12/KCTD16 hetero-oligomers increase the duration of slow IPSCs. In summary, our data demonstrate that simultaneous assembly of distinct KCTDs at the receptor increases the molecular and functional repertoire of native GABAB receptors and modulates physiologically induced K+ current responses in the hippocampus.
Significance statement: The KCTD proteins 8, 12, and 16 are auxiliary subunits of GABAB receptors that differentially regulate G-protein signaling of the receptor. The KCTD proteins are generally assumed to function as homo-oligomers. Here we show that the KCTD proteins also assemble hetero-oligomers in all possible dual combinations. Experiments in live cells demonstrate that KCTD hetero-oligomers form at least tetramers and that these tetramers directly interact with the receptor and the G-protein. KCTD12/KCTD16 hetero-oligomers impart unique kinetic properties to GABAB receptor-induced Kir3 currents in heterologous cells. KCTD12/KCTD16 hetero-oligomers are abundant in the hippocampus, where they prolong the duration of slow IPSCs in pyramidal cells. Our data therefore support that KCTD hetero-oligomers modulate physiologically induced K+ current responses in the brain.
Keywords: G-protein coupled receptor; GABA-B; GPCR; KCTD12; KCTD16; Kir3.
Copyright © 2017 the authors 0270-6474/17/371163-14$15.00/0.
Figures





References
-
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Luján R, Wickman K (2011) Acute cocaine exposure weakens GABAB receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci 31:12251–12257. 10.1523/JNEUROSCI.0494-11.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
