Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP
- PMID: 28003362
- PMCID: PMC5270487
- DOI: 10.1074/jbc.M116.768986
Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP
Abstract
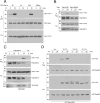
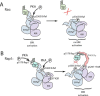
Cyclic adenosine monophosphate (cAMP) is an important mediator of hormonal stimulation of cell growth and differentiation through its activation of the extracellular signal-regulated kinase (ERK) cascade. Two small G proteins, Ras and Rap1 have been proposed to mediate this activation. Using HEK293 cells as a model system, we have recently shown that both Ras and Rap1 are required for cAMP signaling to ERKs. However, cAMP-dependent Ras signaling to ERKs is transient and rapidly terminated by PKA phosphorylation of the Raf isoforms C-Raf and B-Raf. In contrast, cAMP-dependent Rap1 signaling to ERKs and Rap1 is potentiated by PKA. We show that this is due to sustained binding of B-Raf to Rap1. One of the targets of PKA is Rap1 itself, directly phosphorylating Rap1a on serine 180 and Rap1b on serine 179. We show that these phosphorylations create potential binding sites for the adaptor protein 14-3-3 that links Rap1 to the scaffold protein KSR. These results suggest that Rap1 activation of ERKs requires PKA phosphorylation and KSR binding. Because KSR and B-Raf exist as heterodimers within the cell, this binding also brings B-Raf to Rap1, allowing Rap1 to couple to ERKs through B-Raf binding to Rap1 independently of its Ras-binding domain.
Keywords: 14-3-3 protein; Raf kinase; Ras-related protein 1 (Rap1); extracellular-signal-regulated kinase (ERK); protein kinase A (PKA).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Protein Kinase A-independent Ras Protein Activation Cooperates with Rap1 Protein to Mediate Activation of the Extracellular Signal-regulated Kinases (ERK) by cAMP.J Biol Chem. 2016 Oct 7;291(41):21584-21595. doi: 10.1074/jbc.M116.730978. Epub 2016 Aug 16. J Biol Chem. 2016. PMID: 27531745 Free PMC article.
-
Ras-mutant cancer cells display B-Raf binding to Ras that activates extracellular signal-regulated kinase and is inhibited by protein kinase A phosphorylation.J Biol Chem. 2013 Sep 20;288(38):27646-27657. doi: 10.1074/jbc.M113.463067. Epub 2013 Jul 26. J Biol Chem. 2013. PMID: 23893412 Free PMC article.
-
Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation.Mol Cell Biol. 2006 Mar;26(6):2130-45. doi: 10.1128/MCB.26.6.2130-2145.2006. Mol Cell Biol. 2006. PMID: 16507992 Free PMC article.
-
Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation.Trends Cell Biol. 2002 Jun;12(6):258-66. doi: 10.1016/s0962-8924(02)02294-8. Trends Cell Biol. 2002. PMID: 12074885 Review.
-
The Mystery of Rap1 Suppression of Oncogenic Ras.Trends Cancer. 2020 May;6(5):369-379. doi: 10.1016/j.trecan.2020.02.002. Epub 2020 Mar 2. Trends Cancer. 2020. PMID: 32249186 Free PMC article. Review.
Cited by
-
From Physiological Properties to Selective Vulnerability of Motor Units in Amyotrophic Lateral Sclerosis.Adv Neurobiol. 2022;28:375-394. doi: 10.1007/978-3-031-07167-6_15. Adv Neurobiol. 2022. PMID: 36066833
-
Targeting GPCRs and Their Signaling as a Therapeutic Option in Melanoma.Cancers (Basel). 2022 Jan 29;14(3):706. doi: 10.3390/cancers14030706. Cancers (Basel). 2022. PMID: 35158973 Free PMC article. Review.
-
Integration of Rap1 and Calcium Signaling.Int J Mol Sci. 2020 Feb 27;21(5):1616. doi: 10.3390/ijms21051616. Int J Mol Sci. 2020. PMID: 32120817 Free PMC article. Review.
-
Cyclase-associated protein 1 (CAP1) is a prenyl-binding partner of Rap1 GTPase.J Biol Chem. 2018 May 18;293(20):7659-7673. doi: 10.1074/jbc.RA118.001779. Epub 2018 Apr 4. J Biol Chem. 2018. PMID: 29618512 Free PMC article.
-
Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase.Cell Commun Signal. 2023 Jun 14;21(1):136. doi: 10.1186/s12964-023-01146-9. Cell Commun Signal. 2023. PMID: 37316874 Free PMC article.
References
-
- Fujita T., Meguro T., Fukuyama R., Nakamuta H., and Koida M. (2002) New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells: checkpoint of modulation by cyclic AMP. J. Biol. Chem. 277, 22191–22200 - PubMed
-
- Gomez E., Pritchard C., and Herbert T. P. (2002) cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic beta-cells. J. Biol. Chem. 277, 48146–48151 - PubMed
-
- Ehses J. A., Pelech S. L., Pederson R. A., and McIntosh C. H. (2002) Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J. Biol. Chem. 277, 37088–37097 - PubMed
-
- Morozov A., Muzzio I. A., Bourtchouladze R., Van-Strien N., Lapidus K., Yin D., Winder D. G., Adams J. P., Sweatt J. D., and Kandel E. R. (2003) Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron 39, 309–325 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

