IL-10 Receptor Signaling Is Essential for TR1 Cell Function In Vivo
- PMID: 28003377
- PMCID: PMC5263184
- DOI: 10.4049/jimmunol.1601045
IL-10 Receptor Signaling Is Essential for TR1 Cell Function In Vivo
Abstract
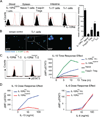
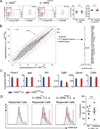
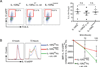
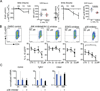
IL-10 is essential to maintain intestinal homeostasis. CD4+ T regulatory type 1 (TR1) cells produce large amounts of this cytokine and are therefore currently being examined in clinical trials as T cell therapy in patients with inflammatory bowel disease. However, factors and molecular signals sustaining TR1 cell regulatory activity still need to be identified to optimize the efficiency and ensure the safety of these trials. We investigated the role of IL-10 signaling in mature TR1 cells in vivo. Double IL-10eGFP Foxp3mRFP reporter mice and transgenic mice with impairment in IL-10 receptor signaling were used to test the activity of TR1 cells in a murine inflammatory bowel disease model, a model that resembles the trials performed in humans. The molecular signaling was elucidated in vitro. Finally, we used human TR1 cells, currently employed for cell therapy, to confirm our results. We found that murine TR1 cells expressed functional IL-10Rα. TR1 cells with impaired IL-10 receptor signaling lost their regulatory activity in vivo. TR1 cells required IL-10 receptor signaling to activate p38 MAPK, thereby sustaining IL-10 production, which ultimately mediated their suppressive activity. Finally, we confirmed these data using human TR1 cells. In conclusion, TR1 cell regulatory activity is dependent on IL-10 receptor signaling. These data suggest that to optimize TR1 cell-based therapy, IL-10 receptor expression has to be taken into consideration.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures


References
-
- Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, Grimbacher B. Infant colitis--it's in the genes. Lancet. 2010;376:1272. - PubMed
-
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361:2033–2045. - PMC - PubMed
-
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

