From the Cover: BDE-47 and BDE-49 Inhibit Axonal Growth in Primary Rat Hippocampal Neuron-Glia Co-Cultures via Ryanodine Receptor-Dependent Mechanisms
- PMID: 28003438
- PMCID: PMC6070026
- DOI: 10.1093/toxsci/kfw259
From the Cover: BDE-47 and BDE-49 Inhibit Axonal Growth in Primary Rat Hippocampal Neuron-Glia Co-Cultures via Ryanodine Receptor-Dependent Mechanisms
Abstract
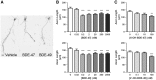
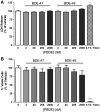
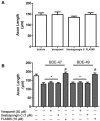
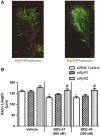
Polybrominated diphenyl ethers (PBDEs) are widespread environmental contaminants associated with adverse neurodevelopmental outcomes in children and preclinical models; however, the mechanisms by which PBDEs cause developmental neurotoxicity remain speculative. The structural similarity between PBDEs and nondioxin-like (NDL) polychlorinated biphenyls (PCBs) suggests shared toxicological properties. Consistent with this, both NDL PCBs and PBDEs have been shown to stabilize ryanodine receptors (RyRs) in the open configuration. NDL PCB effects on RyR activity are causally linked to increased dendritic arborization, but whether PBDEs similarly enhance dendritic growth is not known. In this study, we quantified the effects of individual PBDE congeners on not only dendritic but also axonal growth since both are regulated by RyR-dependent mechanisms, and both are critical determinants of neuronal connectivity. Neuronal-glial co-cultures dissociated from the neonatal rat hippocampus were exposed to BDE-47 or BDE-49 in the culture medium. At concentrations ranging from 20 pM to 2 µM, neither PBDE congener altered dendritic arborization. In contrast, at concentrations ≥ 200 pM, both congeners delayed neuronal polarization resulting in significant inhibition of axonal outgrowth during the first few days in vitro. The axon inhibitory effects of these PBDE congeners occurred independent of cytotoxicity, and were blocked by pharmacological antagonism of RyR or siRNA knockdown of RyR2. These results demonstrate that the molecular and cellular mechanisms by which PBDEs interfere with neurodevelopment overlap with but are distinct from those of NDL PCBs, and suggest that altered patterns of neuronal connectivity may contribute to the developmental neurotoxicity of PBDEs.
Keywords: PBDE; axon; calcium signaling; developmental neurotoxicity; neuronal morphogenesis; ryanodine receptor..
© The Author 2016. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms.Toxicol Sci. 2014 Apr;138(2):379-92. doi: 10.1093/toxsci/kft334. Epub 2014 Jan 2. Toxicol Sci. 2014. PMID: 24385416 Free PMC article.
-
Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity.Environ Health Perspect. 2011 Apr;119(4):519-26. doi: 10.1289/ehp.1002728. Epub 2010 Nov 24. Environ Health Perspect. 2011. PMID: 21106467 Free PMC article.
-
PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms.Environ Health Perspect. 2012 Jul;120(7):997-1002. doi: 10.1289/ehp.1104832. Epub 2012 Apr 25. Environ Health Perspect. 2012. PMID: 22534141 Free PMC article.
-
The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences.Environ Sci Pollut Res Int. 2020 Mar;27(9):8885-8896. doi: 10.1007/s11356-019-06723-5. Epub 2019 Nov 12. Environ Sci Pollut Res Int. 2020. PMID: 31713823 Free PMC article. Review.
-
A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity.Toxicol Lett. 2014 Oct 15;230(2):282-94. doi: 10.1016/j.toxlet.2013.11.011. Epub 2013 Nov 20. Toxicol Lett. 2014. PMID: 24270005 Free PMC article. Review.
Cited by
-
Sex-Dependent Effects of 2,2',3,5',6-Pentachlorobiphenyl on Dendritic Arborization of Primary Mouse Neurons.Toxicol Sci. 2019 Mar 1;168(1):95-109. doi: 10.1093/toxsci/kfy277. Toxicol Sci. 2019. PMID: 30395321 Free PMC article.
-
BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway.Molecules. 2023 Feb 21;28(5):2036. doi: 10.3390/molecules28052036. Molecules. 2023. PMID: 36903282 Free PMC article.
-
PBDE flame retardant exposure causes neurobehavioral and transcriptional effects in first-generation but not second-generation offspring fish.bioRxiv [Preprint]. 2025 May 2:2025.04.28.651134. doi: 10.1101/2025.04.28.651134. bioRxiv. 2025. PMID: 40654795 Free PMC article. Preprint.
-
Detection of 3,3'-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons.Toxicol Sci. 2017 Aug 1;158(2):401-411. doi: 10.1093/toxsci/kfx100. Toxicol Sci. 2017. PMID: 28510766 Free PMC article.
-
Endocrine disruptors also function as nervous disruptors and can be renamed endocrine and nervous disruptors (ENDs).Toxicol Rep. 2021 Jul 31;8:1538-1557. doi: 10.1016/j.toxrep.2021.07.014. eCollection 2021. Toxicol Rep. 2021. PMID: 34430217 Free PMC article. Review.
References
-
- Behl M., Hsieh J. H., Shafer T. J., Mundy W. R., Rice J. R., Boyd W. A., Freedman J. H., Hunter E. S. 3rd, Jarema K. A., Padilla S., et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52, 181–193. - PubMed
-
- Berger-Sweeney J., Hohmann C. F. (1997). Behavioral consequences of abnormal cortical development: Insights into developmental disabilities. Behav. Brain Res. 86, 121–142. - PubMed
-
- Berghuis S. A., Bos A. F., Sauer P. J., Roze E. (2015). Developmental neurotoxicity of persistent organic pollutants: An update on childhood outcome. Arch. Toxicol. 89, 687–709. - PubMed
-
- Berridge M. J. (2006). Calcium microdomains: Organization and function. Cell Calcium 40, 405–412. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

