From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis
- PMID: 28003439
- PMCID: PMC5412087
- DOI: 10.1093/toxsci/kfw261
From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis
Abstract
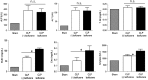
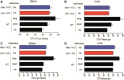
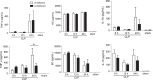
Sepsis continues to result in high morbidity and mortality. General anesthesia is often administered to septic patients, but the impacts of general anesthesia on host defense are not well understood. General anesthesia can be given by volatile and intravenous anesthetics. Our previous in vitro study showed that volatile anesthetic isoflurane directly inhibits leukocyte function-associated antigen-1 (LFA-1) and macrophage-1 antigen (Mac-1), critical adhesion molecules on leukocytes. Thus, the role of isoflurane exposure on in vivo LFA-1 and Mac-1 function was examined using polymicrobial abdominal sepsis model in mice. As a comparison, intravenous anesthetic propofol was given to a group of mice. Wild type, LFA-1, Mac-1, and adhesion molecule-1 knockout mice were used. Following the induction of polymicrobial abdominal sepsis by cecal ligation and puncture, groups of mice were exposed to isoflurane for either 2 or 6 h, or to propofol for 6 h, and their outcomes were examined. Bacterial loads in tissues and blood, neutrophil recruitment to the peritoneal cavity and phagocytosis were studied. Six hours of isoflurane exposure worsened the outcome of abdominal sepsis (P < .0001) with higher bacterial loads in tissues, but 2 h of isoflurane or 6 h of propofol exposure did not. Isoflurane impaired neutrophil recruitment to the abdominal cavity by inhibiting LFA-1 function. Isoflurane also impaired bacterial phagocytosis via complement receptors including Mac-1. In conclusion, prolonged isoflurane exposure worsened the outcome of experimental polymicrobial abdominal sepsis and was associated with impaired neutrophil recruitment and bacterial phagocytosis via reduced LFA-1 and Mac-1 function.
Keywords: anesthesia; leukocyte function-associated antigen-1; macrophage-1 antigen.; neutrophil; sepsis.
© The Author 2016. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Alves-Filho J. C., de Freitas A., Spiller F., Souto F. O., Cunha F. Q. (2008). The role of neutrophils in severe sepsis. Shock 30(Suppl 1), 3–9. - PubMed
-
- Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001). Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310. - PubMed
-
- Bullard D. C., Hu X., Schoeb T. R., Collins R. G., Beaudet A. L., Barnum S. R. (2007). Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J. Immunol. 178, 851–857. - PubMed
-
- Burns J. A., Issekutz T. B., Yagita H., Issekutz A. C. (2001). The alpha 4 beta 1 (very late antigen (VLA)-4, CD49d/CD29) and alpha 5 beta 1 (VLA-5, CD49e/CD29) integrins mediate beta 2 (CD11/CD18) integrin-independent neutrophil recruitment to endotoxin-induced lung inflammation. J. Immunol. 166, 4644–4649. - PubMed
-
- Campagna J. A., Miller K. W., Forman S. A. (2003). Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348, 2110–2124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

