Whole-GUV patch-clamping
- PMID: 28003462
- PMCID: PMC5240670
- DOI: 10.1073/pnas.1609142114
Whole-GUV patch-clamping
Abstract
Studying how the membrane modulates ion channel and transporter activity is challenging because cells actively regulate membrane properties, whereas existing in vitro systems have limitations, such as residual solvent and unphysiologically high membrane tension. Cell-sized giant unilamellar vesicles (GUVs) would be ideal for in vitro electrophysiology, but efforts to measure the membrane current of intact GUVs have been unsuccessful. In this work, two challenges for obtaining the "whole-GUV" patch-clamp configuration were identified and resolved. First, unless the patch pipette and GUV pressures are precisely matched in the GUV-attached configuration, breaking the patch membrane also ruptures the GUV. Second, GUVs shrink irreversibly because the membrane/glass adhesion creating the high-resistance seal (>1 GΩ) continuously pulls membrane into the pipette. In contrast, for cell-derived giant plasma membrane vesicles (GPMVs), breaking the patch membrane allows the GPMV contents to passivate the pipette surface, thereby dynamically blocking membrane spreading in the whole-GMPV mode. To mimic this dynamic passivation mechanism, beta-casein was encapsulated into GUVs, yielding a stable, high-resistance, whole-GUV configuration for a range of membrane compositions. Specific membrane capacitance measurements confirmed that the membranes were truly solvent-free and that membrane tension could be controlled over a physiological range. Finally, the potential for ion transport studies was tested using the model ion channel, gramicidin, and voltage-clamp fluorometry measurements were performed with a voltage-dependent fluorophore/quencher pair. Whole-GUV patch-clamping allows ion transport and other voltage-dependent processes to be studied while controlling membrane composition, tension, and shape.
Keywords: biomimetic system; electrophysiology; giant unilamellar vesicle; lipid–glass interaction; patch clamp.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
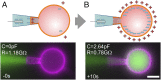


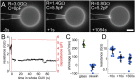
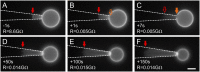
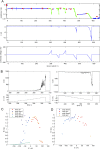

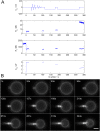


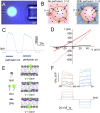

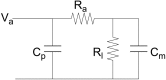
References
-
- Hille B. Ion Channels of Excitable Membranes. 3rd Ed Sinauer; Sunderland, MA: 2001.
-
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391(2):85–100. - PubMed
-
- Varghese A, Tenbroek EM, Coles J, Jr, Sigg DC. Endogenous channels in HEK cells and potential roles in HCN ionic current measurements. Prog Biophys Mol Biol. 2006;90(1-3):26–37. - PubMed
-
- Chandrasekhar KD, Bas T, Kobertz WR. KCNE1 subunits require co-assembly with K+ channels for efficient trafficking and cell surface expression. J Biol Chem. 2006;281(52):40015–40023. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

