Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3
- PMID: 28003463
- PMCID: PMC5240691
- DOI: 10.1073/pnas.1619238114
Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3
Abstract
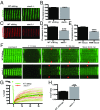
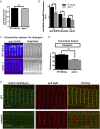
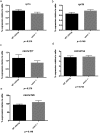
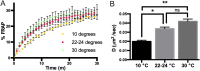
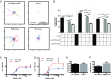
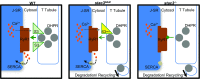
Skeletal muscle contractions are initiated by an increase in Ca2+ released during excitation-contraction (EC) coupling, and defects in EC coupling are associated with human myopathies. EC coupling requires communication between voltage-sensing dihydropyridine receptors (DHPRs) in transverse tubule membrane and Ca2+ release channel ryanodine receptor 1 (RyR1) in the sarcoplasmic reticulum (SR). Stac3 protein (SH3 and cysteine-rich domain 3) is an essential component of the EC coupling apparatus and a mutation in human STAC3 causes the debilitating Native American myopathy (NAM), but the nature of how Stac3 acts on the DHPR and/or RyR1 is unknown. Using electron microscopy, electrophysiology, and dynamic imaging of zebrafish muscle fibers, we find significantly reduced DHPR levels, functionality, and stability in stac3 mutants. Furthermore, stac3NAM myofibers exhibited increased caffeine-induced Ca2+ release across a wide range of concentrations in the absence of altered caffeine sensitivity as well as increased Ca2+ in internal stores, which is consistent with increased SR luminal Ca2+ These findings define critical roles for Stac3 in EC coupling and human disease.
Keywords: Native American myopathy; dihydropyridine receptor; excitation–contraction coupling; skeletal muscle; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

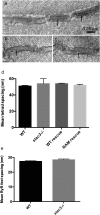
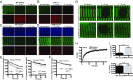
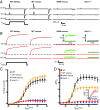
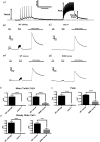
References
-
- Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336(6195):134–139. - PubMed
-
- Lai FA, Erickson HP, Rousseau E, Liu QY, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331(6154):315–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

