Nonstructural Protein 4 of Porcine Reproductive and Respiratory Syndrome Virus Modulates Cell Surface Swine Leukocyte Antigen Class I Expression by Downregulating β2-Microglobulin Transcription
- PMID: 28003480
- PMCID: PMC5309937
- DOI: 10.1128/JVI.01755-16
Nonstructural Protein 4 of Porcine Reproductive and Respiratory Syndrome Virus Modulates Cell Surface Swine Leukocyte Antigen Class I Expression by Downregulating β2-Microglobulin Transcription
Abstract
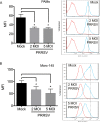
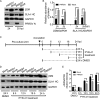
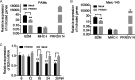
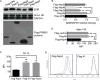
Porcine reproductive and respiratory syndrome virus (PRRSV) is the causative agent of PRRS, which has important impacts on the pig industry. PRRSV infection results in disruption of the swine leukocyte antigen class I (SLA-I) antigen presentation pathway. In this study, highly pathogenic PRRSV (HP-PRRSV) infection inhibited transcription of the β2-microglobulin (β2M) gene (B2M) and reduced cellular levels of β2M, which forms a heterotrimeric complex with the SLA-I heavy chain and a variable peptide and plays a critical role in SLA-I antigen presentation. HP-PRRSV nonstructural protein 4 (Nsp4) was involved in the downregulation of β2M expression. Exogenous expression of Nsp4 downregulated β2M expression at both the mRNA and the protein level and reduced SLA-I expression on the cell surface. Nsp4 bound to the porcine B2M promoter and inhibited its transcriptional activity. Domain III of Nsp4 and the enhancer PAM element of the porcine B2M promoter were identified as essential for the interaction between Nsp4 and B2M These findings demonstrate a novel mechanism whereby HP-PRRSV may modulate the SLA-I antigen presentation pathway and provide new insights into the functions of HP-PRRSV Nsp4. IMPORTANCE PRRSV modulates the host response by disrupting the SLA-I antigen presentation pathway. We show that HP-PRRSV downregulates SLA-I expression on the cell surface via transcriptional inhibition of B2M expression by viral Nsp4. The interaction between domain III of Nsp4 and the enhancer PAM element of the porcine B2M promoter is essential for inhibiting B2M transcription. These observations reveal a novel mechanism whereby HP-PRRSV may modulate SLA-I antigen presentation and provide new insights into the functions of viral Nsp4.
Keywords: Nsp4; PRRSV; SLA-I; porcine B2M promoter; porcine reproductive and respiratory syndrome virus; β2-microglobulin.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Porcine reproductive and respiratory syndrome virus nsp4-mediated β2M downregulation contributes to SLA-I decrease and virus infection in vivo and in vitro.Virology. 2024 Jul;595:110083. doi: 10.1016/j.virol.2024.110083. Epub 2024 Apr 26. Virology. 2024. PMID: 38696887
-
Porcine Reproductive and Respiratory Syndrome Virus Promotes SLA-DR-Mediated Antigen Presentation of Nonstructural Proteins To Evoke a Nonneutralizing Antibody Response In Vivo.J Virol. 2020 Oct 14;94(21):e01423-20. doi: 10.1128/JVI.01423-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796065 Free PMC article.
-
The non-structural protein Nsp2TF of porcine reproductive and respiratory syndrome virus down-regulates the expression of Swine Leukocyte Antigen class I.Virology. 2016 Apr;491:115-24. doi: 10.1016/j.virol.2016.01.021. Epub 2016 Feb 16. Virology. 2016. PMID: 26895249
-
Host microRNAs as regulators of porcine reproductive and respiratory syndrome virus infection.Virology. 2025 Feb;603:110361. doi: 10.1016/j.virol.2024.110361. Epub 2024 Dec 21. Virology. 2025. PMID: 39721195 Review.
-
Mechanisms of suppression of interferon production by porcine reproductive and respiratory syndrome virus.Acta Virol. 2012;56(1):3-9. doi: 10.4149/av_2012_01_3. Acta Virol. 2012. PMID: 22404603 Review.
Cited by
-
Production of Triple-Gene (GGTA1, B2M and CIITA)-Modified Donor Pigs for Xenotransplantation.Front Vet Sci. 2022 Apr 28;9:848833. doi: 10.3389/fvets.2022.848833. eCollection 2022. Front Vet Sci. 2022. PMID: 35573408 Free PMC article.
-
Mapping the Nonstructural Protein Interaction Network of Porcine Reproductive and Respiratory Syndrome Virus.J Virol. 2018 Nov 27;92(24):e01112-18. doi: 10.1128/JVI.01112-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282705 Free PMC article.
-
A Porcine DNMT1 Variant: Molecular Cloning and Generation of Specific Polyclonal Antibody.Genes (Basel). 2023 Jun 23;14(7):1324. doi: 10.3390/genes14071324. Genes (Basel). 2023. PMID: 37510229 Free PMC article.
-
Notch signaling contributes to the expression of inflammatory cytokines induced by highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection in porcine alveolar macrophages.Dev Comp Immunol. 2020 Jul;108:103690. doi: 10.1016/j.dci.2020.103690. Epub 2020 Mar 25. Dev Comp Immunol. 2020. PMID: 32222356 Free PMC article.
-
Mapping the Key Residues within the Porcine Reproductive and Respiratory Syndrome Virus nsp1α Replicase Protein Required for Degradation of Swine Leukocyte Antigen Class I Molecules.Viruses. 2022 Mar 26;14(4):690. doi: 10.3390/v14040690. Viruses. 2022. PMID: 35458420 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

