Residues in the gp41 Ectodomain Regulate HIV-1 Envelope Glycoprotein Conformational Transitions Induced by gp120-Directed Inhibitors
- PMID: 28003492
- PMCID: PMC5309946
- DOI: 10.1128/JVI.02219-16
Residues in the gp41 Ectodomain Regulate HIV-1 Envelope Glycoprotein Conformational Transitions Induced by gp120-Directed Inhibitors
Abstract
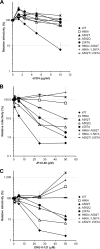
Interactions between the gp120 and gp41 subunits of the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) trimer maintain the metastable unliganded form of the viral spike. Binding of gp120 to the receptor, CD4, changes the Env conformation to promote gp120 interaction with the second receptor, CCR5 or CXCR4. CD4 binding also induces the transformation of Env into the prehairpin intermediate, in which the gp41 heptad repeat 1 (HR1) coiled coil is assembled at the trimer axis. In nature, HIV-1 Envs must balance the requirements to maintain the noncovalent association of gp120 with gp41 and to evade the host antibody response with the need to respond to CD4 binding. Here we show that the gp41 HR1 region contributes to gp120 association with the unliganded Env trimer. Changes in particular amino acid residues in the gp41 HR1 region decreased the efficiency with which Env moved from the unliganded state. Thus, these gp41 changes decreased the sensitivity of HIV-1 to cold inactivation and ligands that require Env conformational changes to bind efficiently. Conversely, these gp41 changes increased HIV-1 sensitivity to small-molecule entry inhibitors that block Env conformational changes induced by CD4. Changes in particular gp41 HR1 amino acid residues can apparently affect the relative stability of the unliganded state and CD4-induced conformations. Thus, the gp41 HR1 region contributes to the association with gp120 and regulates Env transitions from the unliganded state to downstream conformations.IMPORTANCE The development of an efficient vaccine able to prevent HIV infection is a worldwide priority. Knowledge of the envelope glycoprotein structure and the conformational changes that occur after receptor engagement will help researchers to develop an immunogen able to elicit antibodies that block HIV-1 transmission. Here we identify residues in the HIV-1 transmembrane envelope glycoprotein that stabilize the unliganded state by modulating the transitions from the unliganded state to the CD4-bound state.
Keywords: HIV-1; HR1; antibody resistance; gp120; gp41; intrinsic envelope reactivity; soluble CD4.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
HIV-1 gp41 Residues Modulate CD4-Induced Conformational Changes in the Envelope Glycoprotein and Evolution of a Relaxed Conformation of gp120.J Virol. 2018 Jul 31;92(16):e00583-18. doi: 10.1128/JVI.00583-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875245 Free PMC article.
-
Mutations That Increase the Stability of the Postfusion gp41 Conformation of the HIV-1 Envelope Glycoprotein Are Selected by both an X4 and R5 HIV-1 Virus To Escape Fusion Inhibitors Corresponding to Heptad Repeat 1 of gp41, but the gp120 Adaptive Mutations Differ between the Two Viruses.J Virol. 2019 May 15;93(11):e00142-19. doi: 10.1128/JVI.00142-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894471 Free PMC article.
-
Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds.J Virol. 2017 Jan 18;91(3):e01880-16. doi: 10.1128/JVI.01880-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881646 Free PMC article.
-
Progress in targeting HIV-1 entry.Drug Discov Today. 2005 Aug 15;10(16):1085-94. doi: 10.1016/S1359-6446(05)03550-6. Drug Discov Today. 2005. PMID: 16182193 Review.
-
Viral surface glycoproteins, gp120 and gp41, as potential drug targets against HIV-1: brief overview one quarter of a century past the approval of zidovudine, the first anti-retroviral drug.Eur J Med Chem. 2011 Apr;46(4):979-92. doi: 10.1016/j.ejmech.2011.01.046. Epub 2011 Feb 3. Eur J Med Chem. 2011. PMID: 21345545 Review.
Cited by
-
HIV-1-Infected CD4+ T Cells Present MHC Class II-Restricted Epitope via Endogenous Processing.J Immunol. 2022 Sep 1;209(5):864-873. doi: 10.4049/jimmunol.2200145. Epub 2022 Aug 5. J Immunol. 2022. PMID: 36130133 Free PMC article.
-
SOSIP Changes Affect Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Conformation and CD4 Engagement.J Virol. 2018 Sep 12;92(19):e01080-18. doi: 10.1128/JVI.01080-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021898 Free PMC article.
-
Metastable HIV-1 Surface Protein Env Sensitizes Cell Membranes to Transformation and Poration by Dual-Acting Virucidal Entry Inhibitors.Biochemistry. 2020 Feb 18;59(6):818-828. doi: 10.1021/acs.biochem.9b01008. Epub 2020 Jan 28. Biochemistry. 2020. PMID: 31942789 Free PMC article.
-
Asymmetric Structures and Conformational Plasticity of the Uncleaved Full-Length Human Immunodeficiency Virus Envelope Glycoprotein Trimer.J Virol. 2021 Nov 23;95(24):e0052921. doi: 10.1128/JVI.00529-21. Epub 2021 Sep 22. J Virol. 2021. PMID: 34549974 Free PMC article.
-
Assessing bnAb potency in the context of HIV-1 envelope conformational plasticity.PLoS Pathog. 2025 Jan 21;21(1):e1012825. doi: 10.1371/journal.ppat.1012825. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39836706 Free PMC article.
References
-
- Farzan M, Choe H, Desjardins E, Sun Y, Kuhn J, Cao J, Archambault D, Kolchinsky P, Koch M, Wyatt R, Sodroski J. 1998. Stabilization of human immunodeficiency virus type 1 envelope glycoprotein trimers by disulfide bonds introduced into the gp41 glycoprotein ectodomain. J Virol 72:7620–7625. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

