N-Acetyl-L-cysteine Protects the Enterocyte against Oxidative Damage by Modulation of Mitochondrial Function
- PMID: 28003713
- PMCID: PMC5149690
- DOI: 10.1155/2016/8364279
N-Acetyl-L-cysteine Protects the Enterocyte against Oxidative Damage by Modulation of Mitochondrial Function
Abstract
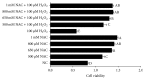
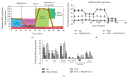
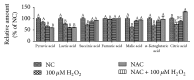
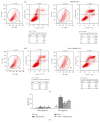
The neonatal small intestine is susceptible to damage caused by oxidative stress. This study aimed to evaluate the protective role of antioxidant N-acetylcysteine (NAC) in intestinal epithelial cells against oxidative damage induced by H2O2. IPEC-J2 cells were cultured in DMEM-H with NAC and H2O2. After 2-day incubation, IPEC-J2 cells were collected for analysis of DNA synthesis, antioxidation capacity, mitochondrial respiration, and cell apoptosis. The results showed that H2O2 significantly decreased (P < 0.05) proliferation rate, mitochondrial respiration, and antioxidation capacity and increased cell apoptosis and the abundance of associated proteins, including cytochrome C, Bcl-XL, cleaved caspase-3, and total caspase-3. NAC supplementation remarkably increased (P < 0.05) proliferation rate, antioxidation capacity, and mitochondrial bioenergetics but decreased cell apoptosis. These findings indicate that NAC might rescue the intestinal injury induced by H2O2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Effects of Quercetin on Proliferation and H₂O₂-Induced Apoptosis of Intestinal Porcine Enterocyte Cells.Molecules. 2018 Aug 12;23(8):2012. doi: 10.3390/molecules23082012. Molecules. 2018. PMID: 30103566 Free PMC article.
-
Carboxymethylated chitosan protects Schwann cells against hydrogen peroxide-induced apoptosis by inhibiting oxidative stress and mitochondria dependent pathway.Eur J Pharmacol. 2018 Apr 15;825:48-56. doi: 10.1016/j.ejphar.2018.02.024. Epub 2018 Feb 17. Eur J Pharmacol. 2018. PMID: 29462593
-
Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis.Sci Rep. 2015 Apr 24;5:9819. doi: 10.1038/srep09819. Sci Rep. 2015. PMID: 25909282 Free PMC article.
-
Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways' response to oxidative stress in weaned piglets.Br J Nutr. 2013 Dec 14;110(11):1938-47. doi: 10.1017/S0007114513001608. Epub 2013 Jun 3. Br J Nutr. 2013. PMID: 23726389 Clinical Trial.
-
Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts.J Alzheimers Dis. 2007 Sep;12(2):195-206. doi: 10.3233/jad-2007-12210. J Alzheimers Dis. 2007. PMID: 17917164 Review.
Cited by
-
Elucidating the mechanisms and mitigation strategies for six-phthalate-induced toxicity in male germ cells.Front Cell Dev Biol. 2024 Jul 10;12:1398176. doi: 10.3389/fcell.2024.1398176. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39050888 Free PMC article.
-
Amino acids regulate energy utilization through mammalian target of rapamycin complex 1 and adenosine monophosphate activated protein kinase pathway in porcine enterocytes.Anim Nutr. 2020 Mar;6(1):98-106. doi: 10.1016/j.aninu.2019.12.001. Epub 2020 Jan 7. Anim Nutr. 2020. PMID: 32211535 Free PMC article.
-
Living biobank-based cancer organoids: prospects and challenges in cancer research.Cancer Biol Med. 2022 Jul 21;19(7):965-82. doi: 10.20892/j.issn.2095-3941.2021.0621. Cancer Biol Med. 2022. PMID: 35856555 Free PMC article. Review.
-
In vitro evidence of antioxidant and anti-inflammatory effects of a new nutraceutical formulation explains benefits in a clinical setting of COPD patients.Front Pharmacol. 2024 Aug 20;15:1439835. doi: 10.3389/fphar.2024.1439835. eCollection 2024. Front Pharmacol. 2024. PMID: 39228520 Free PMC article.
-
Impact of Diquat on the Intestinal Health and the Composition and Function of the Gut Microbiome.Antioxidants (Basel). 2025 Jun 12;14(6):721. doi: 10.3390/antiox14060721. Antioxidants (Basel). 2025. PMID: 40563352 Free PMC article. Review.
References
-
- Tan B., Xiao H., Xiong X., et al. L-arginine improves DNA synthesis in LPS-challenged enterocytes. Frontiers in Bioscience. 2015;20(6):989–1003. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

