Cytotoxic CD4 T Cells: Differentiation, Function, and Application to Dengue Virus Infection
- PMID: 28003809
- PMCID: PMC5141332
- DOI: 10.3389/fimmu.2016.00531
Cytotoxic CD4 T Cells: Differentiation, Function, and Application to Dengue Virus Infection
Abstract
Dengue virus (DENV) has spread through most tropical and subtropical areas of the world and represents a serious public health problem. The control of DENV infection has not yet been fully successful due to lack of effective therapeutics or vaccines. Nevertheless, a better understanding of the immune responses against DENV infection may reveal new strategies for eliciting and improving antiviral immunity. T cells provide protective immunity against various viral infections by generating effector cells that cooperate to eliminate antigens and memory cells that can survive for long periods with enhanced abilities to control recurring pathogens. Following activation, CD8 T cells can migrate to sites of infection and kill infected cells, whereas CD4 T cells contribute to the elimination of pathogens by trafficking to infected tissues and providing help to innate immune responses, B cells, as well as CD8 T cells. However, it is now evident that CD4 T cells can also perform cytotoxic functions and induce the apoptosis of target cells. Importantly, accumulating studies demonstrate that cytotoxic CD4 T cells develop following DENV infections and may play a crucial role in protecting the host from severe dengue disease. We review our current understanding of the differentiation and function of cytotoxic CD4 T cells, with a focus on DENV infection, and discuss the potential of harnessing these cells for the prevention and treatment of DENV infection and disease.
Keywords: CD4 T cells; cytotoxicity; dengue virus; protection; vaccines.
Figures
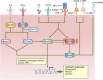
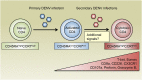
References
-
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet (2012) 380:1559–67. 10.1016/S0140-6736(12)61428-7 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

