Degranulation Response in Cytotoxic Rat Lymphocytes Measured with a Novel CD107a Antibody
- PMID: 28003815
- PMCID: PMC5141239
- DOI: 10.3389/fimmu.2016.00572
Degranulation Response in Cytotoxic Rat Lymphocytes Measured with a Novel CD107a Antibody
Abstract
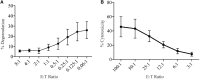
Measuring degranulation through CD107a expression has become an advantageous tool for testing the functional capacity of cytotoxic cells. Such functional studies have been hampered in the rat by the lack of a suitable anti-rat CD107a antibody. In this study, we report a novel hybridoma generated by immunizing Armenian inbred hamsters with transfected Chinese hamster ovary cells expressing CD107a. The SIM1 clone exhibited specific reactivity with CD107a and measured degranulation from natural killer (NK) cells stimulated with target cells or mAb crosslinking of their activating receptors. Degranulation in IL-2-activated NK cells could also be measured, when using low effector to target ratios. SIM1 also stained activated CD8, but not CD4 T cells. This report characterizes the degranulation response in cytotoxic rat cells with a new antibody against rat CD107a.
Keywords: CD107a; cytotoxicity; degranulation; functional assay; lymphocyte; rat.
Figures


References
-
- Masson D, Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem (1985) 260(16):9069–72. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

