Twin arginine translocation system in secretory expression of recombinant human growth hormone
- PMID: 28003839
- PMCID: PMC5168882
- DOI: 10.4103/1735-5362.194871
Twin arginine translocation system in secretory expression of recombinant human growth hormone
Abstract
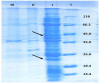
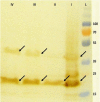
Recombinant protein production in E. coli has several advantages over other expression systems. Misfolding, inclusion body formation, and lack of eukaryotic post translational modification are the most disadvantages of this system. Exporting of correctly folded proteins to the outside of reductive cytoplasmic environment through twin-arginine system could help to pass these limiting steps. Two signal sequences, TorA and SufI are used at N-terminal of human growth hormone (hGH) bearing DsbA gene sequence at C-terminal to enhance folding. The synthetic cassettes including the signal sequence, hGH and DsbA were transformed into E. coli BL21 (DE3) to study the effect of signal sequence and DsbA chaperone on translocation and folding of the protein. The results confirmed using signal sequence at N-terminal of targeted protein and coexpression with DsbA could transport proteins to the periplasmic space and culture media compared to control groups. Although there is no protein band of somatropin in SDS-Page of culture media samples when using SufI as signaling sequence, the study demonstrated TorA signal sequence could transport the target protein to the culture media. However, there was a considerable amount of hGH in periplasmic space when using SufI compared to control.
Keywords: DsbA; Growth hormone; Signal sequence; SufI; TorA; Twin arginine translocation.
Figures




Similar articles
-
Effect of Twine-arginine Translocation-signaling Fusion System and Chaperones Co-expression on Secretory Expression of Somatropin.Adv Biomed Res. 2018 Jan 30;7:17. doi: 10.4103/abr.abr_273_16. eCollection 2018. Adv Biomed Res. 2018. PMID: 29456988 Free PMC article.
-
Specific inhibition of the translocation of a subset of Escherichia coli TAT substrates by the TorA signal peptide.J Mol Biol. 2003 Mar 28;327(3):563-70. doi: 10.1016/s0022-2836(03)00170-0. J Mol Biol. 2003. PMID: 12634052
-
Signal Peptide Hydrophobicity Modulates Interaction with the Twin-Arginine Translocase.mBio. 2017 Aug 1;8(4):e00909-17. doi: 10.1128/mBio.00909-17. mBio. 2017. PMID: 28765221 Free PMC article.
-
Secretory and extracellular production of recombinant proteins using Escherichia coli.Appl Microbiol Biotechnol. 2004 Jun;64(5):625-35. doi: 10.1007/s00253-004-1559-9. Epub 2004 Feb 14. Appl Microbiol Biotechnol. 2004. PMID: 14966662 Review.
-
Secretory production of recombinant proteins in Escherichia coli.Recent Pat Biotechnol. 2010 Jan;4(1):23-9. doi: 10.2174/187220810790069550. Recent Pat Biotechnol. 2010. PMID: 20201800 Review.
Cited by
-
Advancements in Escherichia coli secretion systems for enhanced recombinant protein production.World J Microbiol Biotechnol. 2025 Mar 3;41(3):90. doi: 10.1007/s11274-025-04302-0. World J Microbiol Biotechnol. 2025. PMID: 40025370 Review.
-
Design and production of a novel chimeric human growth hormone superagonist fused to human Fc domain.Res Pharm Sci. 2022 Apr 18;17(3):284-293. doi: 10.4103/1735-5362.343082. eCollection 2022 Jun. Res Pharm Sci. 2022. PMID: 35531129 Free PMC article.
-
Effect of Twine-arginine Translocation-signaling Fusion System and Chaperones Co-expression on Secretory Expression of Somatropin.Adv Biomed Res. 2018 Jan 30;7:17. doi: 10.4103/abr.abr_273_16. eCollection 2018. Adv Biomed Res. 2018. PMID: 29456988 Free PMC article.
-
Growth hormone receptor agonists and antagonists: From protein expression and purification to long-acting formulations.Protein Sci. 2023 Sep;32(9):e4727. doi: 10.1002/pro.4727. Protein Sci. 2023. PMID: 37428391 Free PMC article. Review.
References
-
- Assenberg R, Wan PT, Geisse S, Mayr LM. Advances in recombinant protein expression for use in pharmaceutical research. Curr Opin Struct Biol. 2013;23(3):393–402. - PubMed
-
- Mergulhão FJ, Summers DK, Monteiro GA. Recombinant protein secretion in Escherichia coli. Biotechnol Adv. 2005;23(3):177–202. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

