Paeoniflorin Attenuated Oxidative Stress in Rat COPD Model Induced by Cigarette Smoke
- PMID: 28003846
- PMCID: PMC5149678
- DOI: 10.1155/2016/1698379
Paeoniflorin Attenuated Oxidative Stress in Rat COPD Model Induced by Cigarette Smoke
Abstract
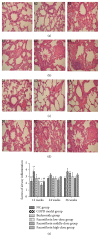
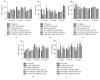
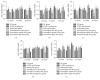
Paeoniflorin (PF), a monoterpene glucoside, might have an effect on the oxidative stress. However, the mechanism is still unknown. In this study, we made the COPD model in Sprague-Dawley (SD) rats by exposing them to the smoke of 20 cigarettes for 1 hour/day and 6 days/week, for 12 weeks, 24 weeks, or 36 weeks. Our findings suggested that smoke inhalation can trigger the oxidative stress from the very beginning. A 24-week treatment of PF especially in the dosage of 40 mg/kg·d can attenuate oxygen stress by partially quenching reactive oxygen species (ROS) and upregulating antioxidant enzymes via an Nrf2-dependent mechanism.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Figures


Similar articles
-
Icaritin attenuates cigarette smoke-mediated oxidative stress in human lung epithelial cells via activation of PI3K-AKT and Nrf2 signaling.Food Chem Toxicol. 2014 Feb;64:307-13. doi: 10.1016/j.fct.2013.12.006. Epub 2013 Dec 8. Food Chem Toxicol. 2014. PMID: 24333105
-
Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis.J Ethnopharmacol. 2016 Jun 5;185:361-9. doi: 10.1016/j.jep.2016.03.031. Epub 2016 Mar 12. J Ethnopharmacol. 2016. PMID: 26979341
-
Vitamin E isoform γ-tocotrienol protects against emphysema in cigarette smoke-induced COPD.Free Radic Biol Med. 2017 Sep;110:332-344. doi: 10.1016/j.freeradbiomed.2017.06.023. Epub 2017 Jul 3. Free Radic Biol Med. 2017. PMID: 28684161
-
Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCδ/NF-κB pathway.Neuroscience. 2015 Jan 29;285:70-80. doi: 10.1016/j.neuroscience.2014.11.008. Epub 2014 Nov 18. Neuroscience. 2015. PMID: 25446358
-
Cigarette Smoke-Induced Acquired Dysfunction of Cystic Fibrosis Transmembrane Conductance Regulator in the Pathogenesis of Chronic Obstructive Pulmonary Disease.Oxid Med Cell Longev. 2018 Apr 23;2018:6567578. doi: 10.1155/2018/6567578. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29849907 Free PMC article. Review.
Cited by
-
Potential Natural Small Molecular Compounds for the Treatment of Chronic Obstructive Pulmonary Disease: An Overview.Front Pharmacol. 2022 Mar 24;13:821941. doi: 10.3389/fphar.2022.821941. eCollection 2022. Front Pharmacol. 2022. PMID: 35401201 Free PMC article. Review.
-
The Pretreatment of Xiaoqinglong Decoction Alleviates Inflammation and Oxidative Damage and Up-Regulates Angiotensin-Converting Enzyme 2 in Lipopolysaccharide-Induced Septic Acute Lung Injury Rats.Evid Based Complement Alternat Med. 2022 Sep 19;2022:2421198. doi: 10.1155/2022/2421198. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36193122 Free PMC article.
-
Efficacy and safety of modified Bushen Yiqi formulas (MBYF) as an add-on to formoterol and budesonide in the management of COPD: study protocol for a multicentre, double-blind, placebo-controlled, parallel-group, randomized clinical trial: FB-MBYF Trial.Trials. 2022 Feb 14;23(1):143. doi: 10.1186/s13063-022-06057-7. Trials. 2022. PMID: 35164853 Free PMC article.
-
Modulating mitochondria with natural extract compounds: from bench to clinical therapeutic opportunities for COPD.Front Pharmacol. 2025 May 21;16:1531302. doi: 10.3389/fphar.2025.1531302. eCollection 2025. Front Pharmacol. 2025. PMID: 40469988 Free PMC article. Review.
-
TCM targets ferroptosis: potential treatments for cancer.Front Pharmacol. 2024 Apr 22;15:1360030. doi: 10.3389/fphar.2024.1360030. eCollection 2024. Front Pharmacol. 2024. PMID: 38738174 Free PMC article. Review.
References
-
- Pauwels R. A., Buist A. S., Calverley P. M., Jenkins C. R., Hurd S. S., GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American Journal of Respiratory and Critical Care Medicine. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. - DOI - PubMed
-
- Zhou Y., Zhang A., Sun H., Yan G., Wang X. Plant-derived natural products as leads to antitumor drugs. Plant Science Today. 2014;1(2):46–61. doi: 10.14719/pst.2014.1.1.17. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

