Virus Infections on Prion Diseased Mice Exacerbate Inflammatory Microglial Response
- PMID: 28003864
- PMCID: PMC5149707
- DOI: 10.1155/2016/3974648
Virus Infections on Prion Diseased Mice Exacerbate Inflammatory Microglial Response
Abstract
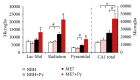
We investigated possible interaction between an arbovirus infection and the ME7 induced mice prion disease. C57BL/6, females, 6-week-old, were submitted to a bilateral intrahippocampal injection of ME7 prion strain (ME7) or normal brain homogenate (NBH). After injections, animals were organized into two groups: NBH (n = 26) and ME7 (n = 29). At 15th week after injections (wpi), animals were challenged intranasally with a suspension of Piry arbovirus 0.001% or with NBH. Behavioral changes in ME7 animals appeared in burrowing activity at 14 wpi. Hyperactivity on open field test, errors on rod bridge, and time reduction in inverted screen were detected at 15th, 19th, and 20th wpi respectively. Burrowing was more sensitive to earlier hippocampus dysfunction. However, Piry-infection did not significantly affect the already ongoing burrowing decline in the ME7-treated mice. After behavioral tests, brains were processed for IBA1, protease-resistant form of PrP, and Piry virus antigens. Although virus infection in isolation did not change the number of microglia in CA1, virus infection in prion diseased mice (at 17th wpi) induced changes in number and morphology of microglia in a laminar-dependent way. We suggest that virus infection exacerbates microglial inflammatory response to a greater degree in prion-infected mice, and this is not necessarily correlated with hippocampal-dependent behavioral deficits.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Age and Environment Influences on Mouse Prion Disease Progression: Behavioral Changes and Morphometry and Stereology of Hippocampal Astrocytes.Oxid Med Cell Longev. 2017;2017:4504925. doi: 10.1155/2017/4504925. Epub 2017 Jan 24. Oxid Med Cell Longev. 2017. PMID: 28243355 Free PMC article.
-
Loss of Perineuronal Net in ME7 Prion Disease.J Neuropathol Exp Neurol. 2008 Mar;67(3):189-99. doi: 10.1097/NEN.0b013e3181654386. J Neuropathol Exp Neurol. 2008. PMID: 18344910
-
Three-dimensional morphometric analysis of microglial changes in a mouse model of virus encephalitis: age and environmental influences.Eur J Neurosci. 2015 Aug;42(4):2036-50. doi: 10.1111/ejn.12951. Epub 2015 Jun 26. Eur J Neurosci. 2015. PMID: 25980955
-
Change in tau phosphorylation associated with neurodegeneration in the ME7 model of prion disease.Biochem Soc Trans. 2010 Apr;38(2):545-51. doi: 10.1042/BST0380545. Biochem Soc Trans. 2010. PMID: 20298219 Review.
-
Cyclooxygenase-2, prostaglandin E2, and microglial activation in prion diseases.Int Rev Neurobiol. 2007;82:265-75. doi: 10.1016/S0074-7742(07)82014-9. Int Rev Neurobiol. 2007. PMID: 17678966 Review.
Cited by
-
The first non-prion pathogen identified: neurotropic influenza virus.Prion. 2022 Dec;16(1):1-6. doi: 10.1080/19336896.2021.2015224. Prion. 2022. PMID: 34978525 Free PMC article.
-
Limbic Encephalitis Brain Damage Induced by Cocal Virus in Adult Mice Is Reduced by Environmental Enrichment: Neuropathological and Behavioral Studies.Viruses. 2020 Dec 30;13(1):48. doi: 10.3390/v13010048. Viruses. 2020. PMID: 33396704 Free PMC article.
-
The Effects of Immune System Modulation on Prion Disease Susceptibility and Pathogenesis.Int J Mol Sci. 2020 Oct 2;21(19):7299. doi: 10.3390/ijms21197299. Int J Mol Sci. 2020. PMID: 33023255 Free PMC article. Review.
-
An Adequate Supply of Bis(ethylmaltolato)oxidovanadium(IV) Remarkably Reversed the Pathological Hallmarks of Alzheimer's Disease in Triple-Transgenic Middle-Aged Mice.Biol Trace Elem Res. 2022 Jul;200(7):3248-3264. doi: 10.1007/s12011-021-02938-1. Epub 2022 Jan 15. Biol Trace Elem Res. 2022. PMID: 35031965
-
Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases.Front Aging Neurosci. 2017 May 15;9:139. doi: 10.3389/fnagi.2017.00139. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28555105 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

