Peginterferon Alfa-2a/Ribavirin treatment efficacy in chronic hepatitis C patients is related to natural killer group 2D gene rs1049174 GC polymorphism
- PMID: 28004016
- PMCID: PMC5142600
- DOI: 10.1007/s13337-016-0349-1
Peginterferon Alfa-2a/Ribavirin treatment efficacy in chronic hepatitis C patients is related to natural killer group 2D gene rs1049174 GC polymorphism
Abstract
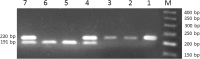
Natural killer group 2D (NKG2D), as an activating receptor, plays pivotal role in viral infectious diseases. Several single nucleotide polymorphisms (SNPs) in the NKG2D gene have characterized that the rs1049174G/C SNP of NKG2D is in the spotlight of notice because of its role in activating of human T cells. This study aimed to investigate rs1049174G/C genetic polymorphism in Chronic Hepatitis C (CHC) patients. The study compromised 107 CHC patients with genotype 1a and 1b. All recruited patients were under treatment with Peginterferon Alfa-2a/Ribavirin according to standard protocol. After completing treatment, 67 patients showed sustained virologic response (SVR) and the rest of patients did not respond to the treatment and considered as non-responder (NR). Genotyping of NKG2D rs1049174G/C SNP was performed using PCR-RFLP method in SVR and NR patients. The NKG2D rs1049174 genotypes frequency for GG, GC and CC were 45, 41 and 14 % respectively. Genotypes distribution were significantly different between SVR and NR groups (p = 0.005). So that the patients with the homozygous GG genotype demonstrated a higher response to Peginterferon Alfa-2a/Ribavirin therapy against HCV infection (OR = 6.0, 95 %CI 1.71-21.08, p = 0.005). In conclusion, the rs1049174 GG genotype of NKG2D receptor is an effective factor in successfully treatment of CHC patients by Peginterferon Alfa-2a/Ribavirin.
Keywords: Chronic hepatitis C; NKG2D; Peginterferon Alfa-2a/Ribavirin; rs1049174.
Figures
Similar articles
-
Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial.Lancet. 2014 Aug 2;384(9941):414-26. doi: 10.1016/S0140-6736(14)60538-9. Epub 2014 Jun 4. Lancet. 2014. PMID: 24907224 Clinical Trial.
-
Randomized controlled trial of danoprevir plus peginterferon alfa-2a and ribavirin in treatment-naïve patients with hepatitis C virus genotype 1 infection.Gastroenterology. 2013 Oct;145(4):790-800.e3. doi: 10.1053/j.gastro.2013.06.051. Epub 2013 Jun 26. Gastroenterology. 2013. PMID: 23811112 Clinical Trial.
-
Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial.Lancet. 2014 Aug 2;384(9941):403-13. doi: 10.1016/S0140-6736(14)60494-3. Epub 2014 Jun 4. Lancet. 2014. PMID: 24907225 Clinical Trial.
-
A comparison of peginterferon α-2a and α-2b for treatment-naive patients with chronic hepatitis C virus: A meta-analysis of randomized trials.Clin Ther. 2010 Aug;32(9):1565-77. doi: 10.1016/j.clinthera.2010.08.009. Clin Ther. 2010. PMID: 20974315 Review.
-
Simeprevir and sofosbuvir for treatment of hepatitis C infection.Am J Health Syst Pharm. 2015 Sep 1;72(17):1445-55. doi: 10.2146/ajhp140290. Am J Health Syst Pharm. 2015. PMID: 26294237 Review.
Cited by
-
NKG2D Natural Killer Cell Receptor-A Short Description and Potential Clinical Applications.Cells. 2021 Jun 7;10(6):1420. doi: 10.3390/cells10061420. Cells. 2021. PMID: 34200375 Free PMC article. Review.
-
The Donor Major Histocompatibility Complex Class I Chain-Related Molecule A Allele rs2596538 G Predicts Cytomegalovirus Viremia in Kidney Transplant Recipients.Front Immunol. 2018 May 8;9:917. doi: 10.3389/fimmu.2018.00917. eCollection 2018. Front Immunol. 2018. PMID: 29867932 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous