The Effect of Aerosol Saline on Laboratory-Induced Dyspnea
- PMID: 28004181
- PMCID: PMC5394930
- DOI: 10.1007/s00408-016-9971-3
The Effect of Aerosol Saline on Laboratory-Induced Dyspnea
Abstract
Purpose: In the 'placebo arm' of a recent study, we found that aerosol saline (sham treatment) produced substantial relief of laboratory-induced dyspnea (Breathing discomfort-BD) in nearly half the subjects. The sham intervention included a physiological change, and instructions to subjects could have produced expectation of dyspnea relief. In the present study, we attempted to discover whether the response to sham aerosol was driven by behavioral or physiological aspects of the intervention.
Methods: Dyspnea (air hunger) was evoked by constraining tidal volume during graded hypercapnia. We measured [Formula: see text] versus BD relationship before and after aerosol saline. To minimize subjects' expectations of dyspnea relief, participants were clearly instructed that we would only deliver saline aerosol. In Protocol 1, we delivered aerosol saline with a ventilator (mimicking our prior study); in Protocol 2, we delivered aerosol without a ventilator.
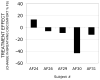
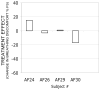
Results: Administration of aerosol saline had little effect on BD in this group of subjects with one exception: one subject experienced appreciable reduction in BD in Protocol 1. This treatment effect was less in Protocol 2. The two most likely explanations are (a) that procedures surrounding ventilator administration of aerosol produced a psychological placebo treatment effect even though the subject knew a drug was not given; (b) there were behavioral changes in breathing undetected by our measurements of respiratory flow and volume that altered the subjects comfort.
Conclusion: When the expectation of treatment effect is minimized, a significant reduction in dyspnea in response to saline placebo is uncommon but not impossible.
Keywords: Aerosol treatment; Dyspnea; Placebo; Symptom management.
Figures


Similar articles
-
Controlled Delivery of 80 mg Aerosol Furosemide Does Not Achieve Consistent Dyspnea Relief in Patients.Lung. 2020 Feb;198(1):113-120. doi: 10.1007/s00408-019-00292-7. Epub 2019 Nov 15. Lung. 2020. PMID: 31728632 Free PMC article. Clinical Trial.
-
Aerosol furosemide for dyspnea: Controlled delivery does not improve effectiveness.Respir Physiol Neurobiol. 2018 Jan;247:146-155. doi: 10.1016/j.resp.2017.10.002. Epub 2017 Oct 12. Respir Physiol Neurobiol. 2018. PMID: 29031573 Free PMC article. Clinical Trial.
-
Aerosol furosemide for dyspnea: High-dose controlled delivery does not improve effectiveness.Respir Physiol Neurobiol. 2018 Jan;247:24-30. doi: 10.1016/j.resp.2017.08.010. Epub 2017 Aug 24. Respir Physiol Neurobiol. 2018. PMID: 28843675 Free PMC article.
-
Asynchrony and dyspnea.Respir Care. 2013 Jun;58(6):973-89. doi: 10.4187/respcare.02507. Respir Care. 2013. PMID: 23709195 Review.
-
Air and soul: the science and application of aerosol therapy.Respir Care. 2010 Jul;55(7):911-21. Respir Care. 2010. PMID: 20587104 Review.
Cited by
-
Placebo and Nocebo Effects Across Symptoms: From Pain to Fatigue, Dyspnea, Nausea, and Itch.Front Psychiatry. 2019 Jul 2;10:470. doi: 10.3389/fpsyt.2019.00470. eCollection 2019. Front Psychiatry. 2019. PMID: 31312148 Free PMC article. Review.
-
Air Hunger: A Primal Sensation and a Primary Element of Dyspnea.Compr Physiol. 2021 Feb 12;11(2):1449-1483. doi: 10.1002/cphy.c200001. Compr Physiol. 2021. PMID: 33577128 Free PMC article.
-
Measuring dyspnoea: new multidimensional instruments to match our 21st century understanding.Eur Respir J. 2017 Mar 2;49(3):1602473. doi: 10.1183/13993003.02473-2016. Print 2017 Mar. Eur Respir J. 2017. PMID: 28254768 Free PMC article. No abstract available.
-
Evaluating the Placebo Status of Nebulized Normal Saline in Patients With Acute Viral Bronchiolitis: A Systematic Review and Meta-analysis.JAMA Pediatr. 2020 Mar 1;174(3):250-259. doi: 10.1001/jamapediatrics.2019.5195. JAMA Pediatr. 2020. PMID: 31905239 Free PMC article.
-
Controlled Delivery of 80 mg Aerosol Furosemide Does Not Achieve Consistent Dyspnea Relief in Patients.Lung. 2020 Feb;198(1):113-120. doi: 10.1007/s00408-019-00292-7. Epub 2019 Nov 15. Lung. 2020. PMID: 31728632 Free PMC article. Clinical Trial.
References
-
- Stone P, Kurowska A, Tookman A. Nebulized frusemide for dyspnoea. Palliat Med. 1994;8(3):258. - PubMed
-
- Moosavi SH, Binks AP, Lansing RW, et al. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir Physiol Neurobiol. 2007;156(1):1–8. - PubMed
-
- Nishino T, Ide T, Sudo T, et al. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am J Respir Crit Care Med. 2000;161(6):1963–1967. - PubMed
-
- Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage. 2002;23(1):73–76. - PubMed
-
- Kohara H, Ueoka H, Aoe K, et al. Effect of nebulized furosemide in terminally ill cancer patients with dyspnea. J Pain Symptom Manage. 2003;26(4):962–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

