Quantitative Analysis of Aquaporin Expression Levels during the Development and Maturation of the Inner Ear
- PMID: 28004290
- PMCID: PMC5352615
- DOI: 10.1007/s10162-016-0607-3
Quantitative Analysis of Aquaporin Expression Levels during the Development and Maturation of the Inner Ear
Abstract
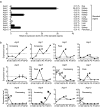
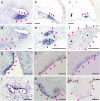
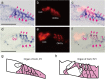
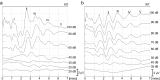
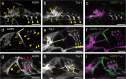
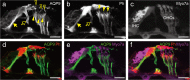
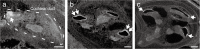
Aquaporins (AQPs) are a family of small membrane proteins that transport water molecules across the plasma membrane along the osmotic gradient. Mammals express 13 subtypes of AQPs, including the recently reported "subcellular AQPs", AQP11 and 12. Each organ expresses specific subsets of AQP subtypes, and in the inner ear, AQPs are essential for the establishment and maintenance of two distinct fluids, endolymph and perilymph. To evaluate the contribution of AQPs during the establishment of inner ear function, we used quantitative reverse transcription polymerase chain reaction to quantify the expression levels of all known AQPs during the entire development and maturation of the inner ear. Using systematic and longitudinal quantification, we found that AQP11 was majorly and constantly expressed in the inner ear, and that the expression levels of several AQPs follow characteristic longitudinal patterns: increasing (Aqp0, 1, and 9), decreasing (Aqp6, 8, and 12), and peak of expression on E18 (Aqp2, 5, and 7). In particular, the expression level of Aqp9 increased by 70-fold during P3-P21. We also performed in situ hybridization of Aqp11, and determined the unique localization of Aqp11 in the outer hair cells. Immunohistochemistry of AQP9 revealed its localization in the supporting cells inside the organ of Corti, and in the root cells. The emergence of AQP9 expression in these cells was during P3-P21, which was coincident with the marked increase of its expression level. Combining these quantification and localization data, we discuss the possible contributions of these AQPs to inner ear function.
Keywords: aquaporin; development; inner ear; qRT-PCR.
Conflict of interest statement
The authors declare no conflict of interest associated with this manuscript.
Figures


Similar articles
-
Expression patterns of aquaporins in the inner ear: evidence for concerted actions of multiple types of aquaporins to facilitate water transport in the cochlea.Hear Res. 2002 Mar;165(1-2):85-95. doi: 10.1016/s0378-5955(02)00288-5. Hear Res. 2002. PMID: 12031518
-
Water channel proteins in the inner ear and their link to hearing impairment and deafness.Mol Aspects Med. 2012 Oct-Dec;33(5-6):612-37. doi: 10.1016/j.mam.2012.06.004. Epub 2012 Jun 23. Mol Aspects Med. 2012. PMID: 22732097 Review.
-
Analysis of aquaporin expression in liver with a focus on hepatocytes.Histochem Cell Biol. 2015 Oct;144(4):347-63. doi: 10.1007/s00418-015-1341-3. Epub 2015 Jul 1. Histochem Cell Biol. 2015. PMID: 26126651
-
Developmental expression of aquaporin 2 in the mouse inner ear.Laryngoscope. 2000 Nov;110(11):1925-30. doi: 10.1097/00005537-200011000-00030. Laryngoscope. 2000. PMID: 11081612
-
Expression of aquaporins in inner ear disease.Laryngoscope. 2020 Jun;130(6):1532-1539. doi: 10.1002/lary.28334. Epub 2019 Oct 8. Laryngoscope. 2020. PMID: 31593306
Cited by
-
Oral exposure to arsenic causes hearing loss in young people aged 12-29 years and in young mice.Sci Rep. 2017 Jul 28;7(1):6844. doi: 10.1038/s41598-017-06096-0. Sci Rep. 2017. PMID: 28754998 Free PMC article.
-
Retinoic acid receptor assembly dynamics governs dual functions in cochlear organogenesis.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2426739122. doi: 10.1073/pnas.2426739122. Epub 2025 Jun 27. Proc Natl Acad Sci U S A. 2025. PMID: 40577120
-
Aquaporin-7: A Dynamic Aquaglyceroporin With Greater Water and Glycerol Permeability Than Its Bacterial Homolog GlpF.Front Physiol. 2020 Jun 30;11:728. doi: 10.3389/fphys.2020.00728. eCollection 2020. Front Physiol. 2020. PMID: 32695023 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

