A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies
- PMID: 28004361
- PMCID: PMC5331088
- DOI: 10.1007/s12325-016-0451-1
A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies
Abstract
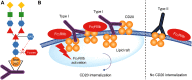
Obinutuzumab (GA101) is a novel, type II, glycoengineered, humanized anti-CD20 monoclonal antibody that has been developed to address the need for new therapeutics with improved efficacy in patients with lymphocytic leukemia and lymphoma of B-cell origin. Obinutuzumab has a distinct mode of action relative to type I anti-CD20 antibodies, such as rituximab, working primarily by inducing direct cell death and antibody-dependent cell-mediated cytotoxicity. Obinutuzumab is under investigation in a wide-ranging program of clinical trials in patients with B-cell malignancies. Efficacy as monotherapy has been reported in patients with relapsed/refractory indolent and aggressive non-Hodgkin lymphoma (NHL) and in chronic lymphocytic leukemia (CLL) of B-cell origin. Improved outcomes have also been noted when obinutuzumab is added to chemotherapy in patients with B-cell NHL, and superiority over rituximab has been reported with combination therapy in patients with CLL. Ongoing research is focusing on developing options for chemotherapy-free treatment and on new combinations of obinutuzumab with novel targeted agents.
Keywords: Antibody-dependent cell-mediated cytotoxicity; B-cell lymphoma; CD20; Chronic lymphocytic leukemia; Glycoengineering; Monoclonal antibody; Non-Hodgkin lymphoma; Obinutuzumab; Oncology; Rituximab.
Figures

References
-
- American Cancer Society. What is chronic lymphocytic leukemia? 2016. http://www.cancer.org/cancer/leukemia-chroniclymphocyticcll/detailedguid.... Accessed 20 July 2016.
-
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v78–v84. - PubMed
-
- Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissue (IARC WHO classification of tumours). 4th ed. Geneva: World Health Organization; 2008.
-
- Lymphoma Research Foundation. About lymphoma. 2016. http://www.lymphoma.org/site/pp.asp?c=bkLTKaOQLmK8E&b=6299689. Accessed 20 July 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

