Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats
- PMID: 28004411
- PMCID: PMC5350477
- DOI: 10.1113/JP273335
Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats
Abstract
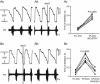
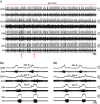
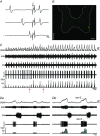
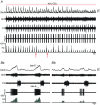
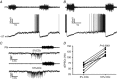
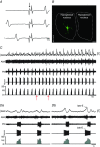
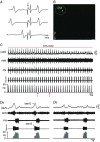
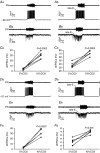
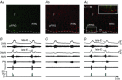
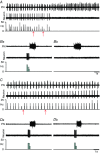
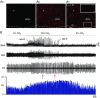
Key points: Hypercapnia or parafacial respiratory group (pFRG) disinhibition at normocapnia evokes active expiration in rats by recruitment of pFRG late-expiratory (late-E) neurons. We show that hypercapnia simultaneously evoked active expiration and exaggerated glottal dilatation by late-E synaptic excitation of abdominal, hypoglossal and laryngeal motoneurons. Simultaneous rhythmic expiratory activity in previously silent pFRG late-E neurons, which did not express the marker of ventral medullary CO2 -sensitive neurons (transcription factor Phox2b), was also evoked by hypercapnia. Hypercapnia-evoked active expiration, neural and neuronal late-E activities were eliminated by pFRG inhibition, but not after blockade of synaptic excitation. Hypercapnia produces disinhibition of non-chemosensitive pFRG late-E neurons to evoke active expiration and concomitant cranial motor respiratory responses controlling the oropharyngeal and upper airway patency.
Abstract: Hypercapnia produces active expiration in rats and the recruitment of late-expiratory (late-E) neurons located in the parafacial respiratory group (pFRG) of the ventral medullary brainstem. We tested the hypothesis that hypercapnia produces active expiration and concomitant cranial respiratory motor responses controlling the oropharyngeal and upper airway patency by disinhibition of pFRG late-E neurons, but not via synaptic excitation. Phrenic nerve, abdominal nerve (AbN), cranial respiratory motor nerves, subglottal pressure, and medullary and spinal neurons/motoneurons were recorded in in situ preparations of juvenile rats. Hypercapnia evoked AbN active expiration, exaggerated late-E discharges in cranial respiratory motor outflows, and glottal dilatation via late-E synaptic excitation of abdominal, hypoglossal and laryngeal motoneurons. Simultaneous rhythmic late-E activity in previously silent pFRG neurons, which did not express the marker of ventral medullary CO2 -sensitive neurons (transcription factor Phox2b), was also evoked by hypercapnia. In addition, hypercapnia-evoked AbN active expiration, neural and neuronal late-E activities were eliminated by pFRG inhibition, but not after blockade of synaptic excitation. On the other hand, pFRG inhibition did not affect either hypercapnia-induced inspiratory increases in respiratory motor outflows or CO2 sensitivity of the more medial Phox2b-positive neurons in the retrotrapezoid nucleus (RTN). Our data suggest that neither RTN Phox2b-positive nor other CO2 -sensitive brainstem neurons activate Phox2b-negative pFRG late-E neurons under hypercapnia to produce AbN active expiration and concomitant cranial motor respiratory responses controlling the oropharyngeal and upper airway patency. Hypercapnia produces disinhibition of non-chemosensitive pFRG late-E neurons in in situ preparations of juvenile rats to activate abdominal, hypoglossal and laryngeal motoneurons.
Keywords: abdominal active expiration; airway patency; late-expiratory neurons; parafacial respiratory group; respiratory motoneurons.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

