Moving towards a reliable HIV incidence test - current status, resources available, future directions and challenges ahead
- PMID: 28004622
- PMCID: PMC9507805
- DOI: 10.1017/S0950268816002910
Moving towards a reliable HIV incidence test - current status, resources available, future directions and challenges ahead
Abstract
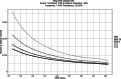
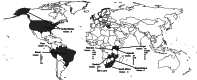
In 2011 the Incidence Assay Critical Path Working Group reviewed the current state of HIV incidence assays and helped to determine a critical path to the introduction of an HIV incidence assay. At that time the Consortium for Evaluation and Performance of HIV Incidence Assays (CEPHIA) was formed to spur progress and raise standards among assay developers, scientists and laboratories involved in HIV incidence measurement and to structure and conduct a direct independent comparative evaluation of the performance of 10 existing HIV incidence assays, to be considered singly and in combinations as recent infection test algorithms. In this paper we report on a new framework for HIV incidence assay evaluation that has emerged from this effort over the past 5 years, which includes a preliminary target product profile for an incidence assay, a consensus around key performance metrics along with analytical tools and deployment of a standardized approach for incidence assay evaluation. The specimen panels for this evaluation have been collected in large volumes, characterized using a novel approach for infection dating rules and assembled into panels designed to assess the impact of important sources of measurement error with incidence assays such as viral subtype, elite host control of viraemia and antiretroviral treatment. We present the specific rationale for several of these innovations, and discuss important resources for assay developers and researchers that have recently become available. Finally, we summarize the key remaining steps on the path to development and implementation of reliable assays for monitoring HIV incidence at a population level.
Keywords: HIV/AIDS; incidence.
Conflict of interest statement
None.
Figures

References
-
- Brookmeyer R, Quinn TC. Estimation of current human immunodeficiency virus incidence rates from a cross-sectional survey using early diagnostic tests. American Journal of Epidemiology 1995; 141: 166–172. - PubMed
-
- Busch MP, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 2010; 24: 2763–2771. - PubMed
-
- Janssen RS, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. Journal of the American Medical Association 1998; 280: 42–48. - PubMed
-
- Parekh BS, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Research and Human Retroviruses 2002; 18: 295–307. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

