Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord
- PMID: 28004735
- PMCID: PMC5177930
- DOI: 10.1038/srep38746
Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord
Abstract
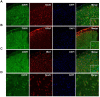
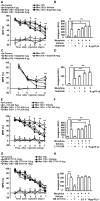
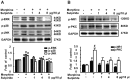
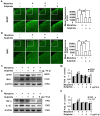
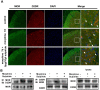
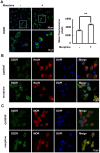
Tolerance induced by morphine remains a major unresolved problem and significantly limits its clinical use. Recent evidences have indicated that dopamine D2 receptor (D2DR) is likely to be involved in morphine-induced antinociceptive tolerance. However, its exact effect and molecular mechanism remain unknown. In this study we examined the effect of D2DR on morphine antinociceptive tolerance in mice spinal cord. Chronic morphine treatment significantly increased levels of D2DR in mice spinal dorsal horn. And the immunoreactivity of D2DR was newly expressed in neurons rather than astrocytes or microglia both in vivo and in vitro. Blockade of D2DR with its antagonist (sulpiride and L-741,626, i.t.) attenuated morphine antinociceptive tolerance without affecting basal pain perception. Sulpiride (i.t.) also down-regulated the expression of phosphorylation of NR1, PKC, MAPKs and suppressed the activation of astrocytes and microglia induced by chronic morphine administration. Particularly, D2DR was found to interact with μ opioid receptor (MOR) in neurons, and chronic morphine treatment enhanced the MOR/D2DR interactions. Sulpiride (i.t.) could disrupt the MOR/D2DR interactions and attenuate morphine tolerance, indicating that neuronal D2DR in the spinal cord may be involved in morphine tolerance possibly by interacting with MOR. These results may present new opportunities for the treatment and management of morphine-induced antinociceptive tolerance which often observed in clinic.
Figures

Similar articles
-
Selective blockade of spinal D2DR by levo-corydalmine attenuates morphine tolerance via suppressing PI3K/Akt-MAPK signaling in a MOR-dependent manner.Exp Mol Med. 2018 Nov 14;50(11):1-12. doi: 10.1038/s12276-018-0175-1. Exp Mol Med. 2018. PMID: 30429454 Free PMC article.
-
The dopamine D1-D2DR complex in the rat spinal cord promotes neuropathic pain by increasing neuronal excitability after chronic constriction injury.Exp Mol Med. 2021 Feb;53(2):235-249. doi: 10.1038/s12276-021-00563-5. Epub 2021 Feb 9. Exp Mol Med. 2021. PMID: 33558591 Free PMC article.
-
Dopamine receptor mechanism(s) and morphine tolerance in mice.J Psychopharmacol. 2002 Sep;16(3):261-6. doi: 10.1177/026988110201600312. J Psychopharmacol. 2002. PMID: 12236635
-
μ Opioid Receptor-Triggered Notch-1 Activation Contributes to Morphine Tolerance: Role of Neuron-Glia Communication.Mol Neurobiol. 2020 Jan;57(1):331-345. doi: 10.1007/s12035-019-01706-6. Epub 2019 Jul 25. Mol Neurobiol. 2020. PMID: 31347026
-
NMDA receptors are involved in upstream of the spinal JNK activation in morphine antinociceptive tolerance.Neurosci Lett. 2009 Dec 25;467(2):95-9. doi: 10.1016/j.neulet.2009.10.013. Epub 2009 Oct 8. Neurosci Lett. 2009. PMID: 19818835
Cited by
-
Blockade of spinal dopamine D1/D2 receptor heteromers by levo-Corydalmine suppressed calcium signaling cascade in spinal neurons to alleviate bone cancer pain in rats.J Cancer. 2024 Jan 1;15(4):1041-1052. doi: 10.7150/jca.91129. eCollection 2024. J Cancer. 2024. PMID: 38230224 Free PMC article.
-
Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor.Biomolecules. 2019 Aug 14;9(8):368. doi: 10.3390/biom9080368. Biomolecules. 2019. PMID: 31416253 Free PMC article.
-
Potentiation of Morphine-Induced Antinociception by Propranolol: The Involvement of Dopamine and GABA Systems.Front Pharmacol. 2017 Nov 10;8:794. doi: 10.3389/fphar.2017.00794. eCollection 2017. Front Pharmacol. 2017. PMID: 29209205 Free PMC article.
-
Exploring Nepicastat Activity: Beyond DβH.Int J Mol Sci. 2025 May 3;26(9):4356. doi: 10.3390/ijms26094356. Int J Mol Sci. 2025. PMID: 40362592 Free PMC article.
-
Sexual dimorphism in striatal dopaminergic responses promotes monogamy in social songbirds.Elife. 2017 Aug 11;6:e25819. doi: 10.7554/eLife.25819. Elife. 2017. PMID: 28826502 Free PMC article.
References
-
- Shimoyama N., Shimoyama M., Davis A. M., Monaghan D. T. & Inturrisi C. E. An antisense oligonucleotide to the N-methyl-D-aspartate (NMDA) subunit NMDAR1 attenuates NMDA-induced nociception, hyperalgesia, and morphine tolerance. The Journal of pharmacology and experimental therapeutics 312, 834–840, doi: 10.1124/jpet.104.074856 (2005). - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

