Investigating the Neuroprotective Effects of Turmeric Extract: Structural Interactions of β-Amyloid Peptide with Single Curcuminoids
- PMID: 28004737
- PMCID: PMC5177957
- DOI: 10.1038/srep38846
Investigating the Neuroprotective Effects of Turmeric Extract: Structural Interactions of β-Amyloid Peptide with Single Curcuminoids
Abstract
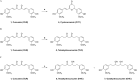
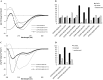
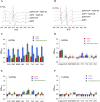
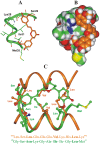
A broad biophysical analysis was performed to investigate the molecular basis of the neuroprotective action of Curcuma longa extracts in Alzheimer's disease. By combining circular dichroism and electron paramagnetic resonance experiments with molecular modeling calculations, the minor components of Curcuma longa extracts, such as demethoxycurcumin (2, DMC), bisdemethoxycurcumin (3, BDMC) and cyclocurcumin (4, CYC), were analyzed in a membrane environment mimicking the phospholipid bilayer. Our study provides the first evidence on the relative role of single curcuminoids interacting with Aβ-peptide. When the CYC and curcumin metabolite tetrahydrocurcumin (5, THC) were inserted into an anionic lipid solution, a significant modification of the Aβ CD curves was detected. These data were implemented by EPR experiments, demonstrating that CYC reaches the inner part of the bilayer, while the other curcuminoids are localized close to the membrane interface. Computational studies provided a model for the curcuminoid-Aβ interaction, highlighting the importance of a constrained "semi-folded" conformation to interact with Aβ analogously to the pattern observed in α-helical coiled-coil peptide structures. This combined approach led to a better understanding of the intriguing in vitro and in vivo activity of curcuminoids as anti-Alzheimer agents, paving a new path for the rational design of optimized druggable analogues.
Figures


References
-
- Selkoe D. J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev 81, 741–766 (2001). - PubMed
-
- Walsh D. M. & Selkoe D. J. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44, 181–193 (2004). - PubMed
-
- Sipe J. D. & Cohen A. S. Review: History of the amyloid fibril. J Struct Biol 130, 88–98 (2000). - PubMed
-
- Hardy J. & Selkoe D. J. Medicine - The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

