Hydrogen sulfide - cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots
- PMID: 28004782
- PMCID: PMC5177925
- DOI: 10.1038/srep39702
Hydrogen sulfide - cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots
Abstract
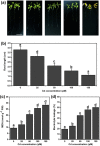
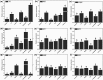
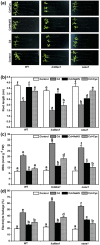
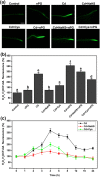
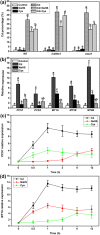
Cadmium (Cd2+) is a common toxic heavy metal ion. We investigated the roles of hydrogen sulfide (H2S) and cysteine (Cys) in plant responses to Cd2+ stress. The expression of H2S synthetic genes LCD and DES1 were induced by Cd2+ within 3 h, and endogenous H2S was then rapidly released. H2S promoted the expression of Cys synthesis-related genes SAT1 and OASA1, which led to endogenous Cys accumulation. The H2S and Cys cycle system was stimulated by Cd2+ stress, and it maintained high levels in plant cells. H2S inhibited the ROS burst by inducing alternative respiration capacity (AP) and antioxidase activity. H2S weakened Cd2+ toxicity by inducing the metallothionein (MTs) genes expression. Cys promoted GSH accumulation and inhibited the ROS burst, and GSH induced the expression of phytochelatin (PCs) genes, counteracting Cd2+ toxicity. In summary, the H2S and Cys cycle system played a key role in plant responses to Cd2+ stress. The Cd2+ tolerance was weakened when the cycle system was blocked in lcddes1-1 and oasa1 mutants. This paper is the first to describe the role of the H2S and Cys cycle system in Cd2+ stress and to explore the relevant and specificity mechanisms of H2S and Cys in mediating Cd2+ stress.
Figures



References
-
- Bolan N. S. et al. Chapter Four—Cadmium Contamination and Its Risk Management in Rice. Ecosystems Adv. Agron. 119, 183–273 (2013).
-
- Sun H. et al. Association of cadmium in urine and blood with age in a general population with low environmental exposure. Chemosphere. 156, 392–397 (2016). - PubMed
-
- Kan Q. et al. Nitrate reductase-mediated NO production enhances Cd accumulation in Panax notoginseng roots by affecting root cell wall properties. J. Plant Physiol. 193, 64–70 (2016). - PubMed
-
- Sandalio L. M., Dalurzo H. C., Gómez M., Romero-Puertas M. C. & del Río L. A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 52, 2115–2126 (2001). - PubMed
-
- Ortega-Villasante C., Rellán-Álvarez R., Del Campo F. F., Carpena-Ruiz R. O. & Hernández L. E. Cellular damage induced by cadmium and mercury in Medicago sativa. J. Exp. Bot. 56, 2239–2251 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

