New Formyl Phloroglucinol Meroterpenoids from the Leaves of Eucalyptus robusta
- PMID: 28004790
- PMCID: PMC5177953
- DOI: 10.1038/srep39815
New Formyl Phloroglucinol Meroterpenoids from the Leaves of Eucalyptus robusta
Abstract
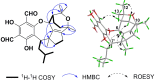
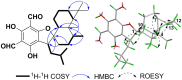
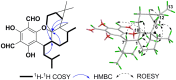
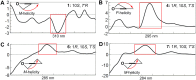
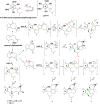
Seven new formyl phloroglucinol meroterpenoids (FPMs), namely eucalrobusones J-P (1-7), as well as three known ones (8-10) were isolated from the leaves of Eucalyptus robusta. Their structures were elucidated by spectroscopic data analysis, and their absolute configurations were determined by applications of the Snatzke's helicity rule and the electron circular dichroism (ECD) calculation. These FPMs are diverse in coupling patterns between phloroglucinol and sesquiterpenoid units, forming novel polycyclic ring systems. Compound 1 possesses a new carbon skeleton that a 1-oxaspiro[5.6]dodecane core is formed through C-14 rather than C-4 of the aromadendrane moiety. Compound 2 features a novel 6/7/5 ring-fused 6-oxabicyclo[3.2.2]nonane skeleton. Compounds 3-5 are rare aristolane-based FPMs. By forming different oxo bridges, compound 3 is the first sample of FPM with benzo-dihydrofuran structure, and compound 4 possesses a novel 6/6/6/6/3-fused pentacyclic skeleton. Compounds 1, 6, and 8 exhibited significant antifungal activities against Candida glabrata with MIC50 values of 2.57, 1.95, and 2.49 μg/mL, respectively.
Figures

References
-
- Nishizawa M. et al. Macrocarpals: HIV-RTase inhibitors of Eucalyptus globulus. Tetrahedron Lett. 33, 2983–2986 (1992).
-
- Shou Q. Y. et al. Rhodomyrtals A-D, four unusual phloroglucinol-sesquiterpene adducts from Rhodomyrtus psidioides. RSC Adv. 4, 13514–13517 (2014).
-
- Yang S. P. et al. Potent HGF/c-Met axis inhibitors from Eucalyptus globulus: the coupling of phloroglucinol and sesquiterpenoid is essential for the activity. J. Med. Chem. 55, 8183–8187 (2012). - PubMed
-
- Tanaka T., Mikamiyama H., Maeda K. & Iwata C. Total synthesis of (−)-macrocarpal C. Stereoselective coupling reaction with a novel hexasubstituted benzene Cr(CO)3 complex as a biomimetic chiral benzyl cation equivalent. J. Org. Chem. 63, 9782–9793 (1998).
-
- Singh I. P., Sidana J., Bharatea S. B. & Foley W. J. Phloroglucinol compounds of natural origin: synthetic aspects. Nat. Prod. Rep. 27, 393–416 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

