Coupled ATPase-adenylate kinase activity in ABC transporters
- PMID: 28004795
- PMCID: PMC5192220
- DOI: 10.1038/ncomms13864
Coupled ATPase-adenylate kinase activity in ABC transporters
Abstract
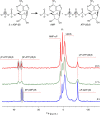
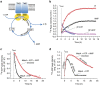
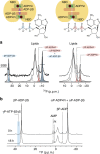
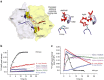
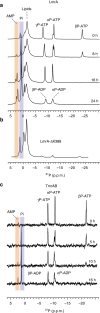
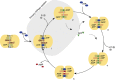
ATP-binding cassette (ABC) transporters, a superfamily of integral membrane proteins, catalyse the translocation of substrates across the cellular membrane by ATP hydrolysis. Here we demonstrate by nucleotide turnover and binding studies based on 31P solid-state NMR spectroscopy that the ABC exporter and lipid A flippase MsbA can couple ATP hydrolysis to an adenylate kinase activity, where ADP is converted into AMP and ATP. Single-point mutations reveal that both ATPase and adenylate kinase mechanisms are associated with the same conserved motifs of the nucleotide-binding domain. Based on these results, we propose a model for the coupled ATPase-adenylate kinase mechanism, involving the canonical and an additional nucleotide-binding site. We extend these findings to other prokaryotic ABC exporters, namely LmrA and TmrAB, suggesting that the coupled activities are a general feature of ABC exporters.
Figures


References
-
- Higgins C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992). - PubMed
-
- Higgins C. F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 446, 749–757 (2007). - PubMed
-
- Dawson R. J. & Locher K. P. Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

