The differentiated airway epithelium infected by influenza viruses maintains the barrier function despite a dramatic loss of ciliated cells
- PMID: 28004801
- PMCID: PMC5177954
- DOI: 10.1038/srep39668
The differentiated airway epithelium infected by influenza viruses maintains the barrier function despite a dramatic loss of ciliated cells
Abstract
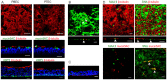
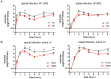
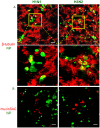
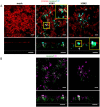
Virus-host interactions in the respiratory epithelium during long term influenza virus infection are not well characterized. Therefore, we developed an air-liquid interface culture system for differentiated porcine respiratory epithelial cells to study the effect of virus-induced cellular damage. In our well-differentiated cells, α2,6-linked sialic acid is predominantly expressed on the apical surface and the basal cells mainly express α2,3-linked sialic acid. During the whole infection period, release of infectious virus was maintained at a high titre for more than seven days. The infected epithelial cells were subject to apoptosis resulting in the loss of ciliated cells together with a thinner thickness. Nevertheless, the airway epithelium maintained trans-epithelial electrical resistance and retained its barrier function. The loss of ciliated cells was compensated by the cells which contained the KRT5 basal cell marker but were not yet differentiated into ciliated cells. These specialized cells showed an increase of α2,3-linked sialic acid on the apical surface. In sum, our results help to explain the localized infection of the airway epithelium by influenza viruses. The impairment of mucociliary clearance in the epithelial cells provides an explanation why prior viral infection renders the host more susceptible to secondary co-infection by another pathogen.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

