Diastereoselective Synthesis of Highly Substituted Tetrahydrofurans by Pd-Catalyzed Tandem Oxidative Cyclization-Redox Relay Reactions Controlled by Intramolecular Hydrogen Bonding
- PMID: 28004933
- PMCID: PMC5224347
- DOI: 10.1021/acs.joc.6b02053
Diastereoselective Synthesis of Highly Substituted Tetrahydrofurans by Pd-Catalyzed Tandem Oxidative Cyclization-Redox Relay Reactions Controlled by Intramolecular Hydrogen Bonding
Abstract
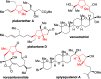
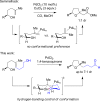
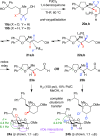
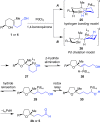
Palladium-catalyzed oxidative cyclization of alkenols provides a convenient entry into cyclic ethers but typically proceeds with little or no diastereoselectivity for cyclization of trisubstituted olefins to form tetrahydrofurans due to the similar energies of competing 5-membered transition-state conformations. Herein, a new variant of this reaction has been developed in which a PdCl2/1,4-benzoquinone catalyst system coupled with introduction of a hydrogen-bond acceptor in the substrate enhances both diastereoselectivity and reactivity. Cyclization occurs with 5-exo Markovnikov regioselectivity. Mechanistic and computational studies support an anti-oxypalladation pathway in which intramolecular hydrogen bonding increases the nucleophilicity of the alcohol and enforces conformational constraints that enhance diastereoselectivity. The cyclization is followed by a tandem redox-relay process that provides versatile side-chain functionalities for further derivatization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
-
For selected examples, see:
- Bowden B. F.; Coll J. C.; Mitchell S. J.; Mulder J.; Stokie G. J. Aust. J. Chem. 1978, 31, 2049–2056. 10.1071/CH9782049. - DOI
- Kamel H. N.; Fronczek F. R.; Khalifa S. I.; Slattery M. Chem. Pharm. Bull. 2007, 55, 537–540. 10.1248/cpb.55.537. - DOI - PubMed
- Sato A.; Fenical W.; Qi-tai Z.; Clardy J. Tetrahedron 1985, 41, 4303–4308. 10.1016/S0040-4020(01)97201-1. - DOI
- Shoji N.; Umeyama A.; Arihara S. J. Nat. Prod. 1993, 56, 1651–1653. 10.1021/np50099a035. - DOI
- Sheu J.-H.; Ahmed A. F.; Shiue R.-T.; Dai C.-F.; Kuo Y.-H. J. Nat. Prod. 2002, 65, 1904–1908. 10.1021/np020280r. - DOI - PubMed
- Ahmed A. F.; Shiue R.-T.; Wang G.-H.; Dai C.-F.; Kuo Y.-H.; Sheu J.-H. Tetrahedron 2003, 59, 7337–7344. 10.1016/S0040-4020(03)01138-4. - DOI
- Rudi A.; Shmul G.; Benayahu Y.; Kashman Y. Tetrahedron Lett. 2006, 47, 2937–2939. 10.1016/j.tetlet.2006.02.118. - DOI
- Kamel H. N.; Ferreira D.; Garcia-Fernandez L. F.; Slattery M. J. Nat. Prod. 2007, 70, 1223–1227. 10.1021/np070074p. - DOI - PubMed
- Takaki H.; Koganemaru R.; Iwakawa Y.; Higuchi R.; Miyamoto T. Biol. Pharm. Bull. 2003, 26, 380–382. 10.1248/bpb.26.380. - DOI - PubMed
-
-
-
For selected examples, see:
- Patil A. D.; Freyer A. J.; Bean M. F.; Carte B. K.; Westley J. W.; Johnson R. K. Tetrahedron 1996, 52, 377–394. 10.1016/0040-4020(95)00856-X. - DOI
- Caffieri F.; Fattorusso E.; Taglialatela-Scafati O.; Ianaro A. Tetrahedron 1999, 55, 7045–7056. 10.1016/S0040-4020(99)00332-4. - DOI
- Caffieri F.; Fattorusso E.; Taglialatela-Scafati O.; Di Rosa M.; Ianaro A. Tetrahedron 1999, 55, 13831–13840. 10.1016/S0040-4020(99)00866-2. - DOI
- Gochfeld D. J.; Hamann M. T. J. Nat. Prod. 2001, 64, 1477–1479. 10.1021/np010216u. - DOI - PubMed
- Campagnuolo C.; Fattorusso E.; Taglialatela-Scafati O.; Ianaro A.; Pisano B. Eur. J. Org. Chem. 2002, 2002, 61–69. 10.1002/1099-0690(20021)2002:1<61::AID-EJOC61>3.0.CO;2-E. - DOI - PubMed
-
-
- Sakemi S.; Higa T.; Jefford C. W.; Bernardinelli G. Tetrahedron Lett. 1986, 27, 4287–4290. 10.1016/S0040-4039(00)94254-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

