Cooperative Brønsted Acid-Type Organocatalysis for the Stereoselective Synthesis of Deoxyglycosides
- PMID: 28004941
- PMCID: PMC5309864
- DOI: 10.1021/acs.joc.6b02498
Cooperative Brønsted Acid-Type Organocatalysis for the Stereoselective Synthesis of Deoxyglycosides
Abstract
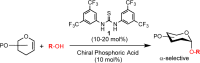
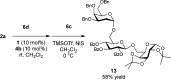
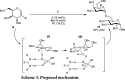
A practical approach for the α-stereoselective synthesis of deoxyglycosides using cooperative Brønsted acid-type organocatalysis has been developed. The method is tolerant of a wide range of glycoside donors and acceptors, and its versatility is exemplified in the one-pot synthesis of a trisaccharide. Mechanistic studies suggest that thiourea-induced acid amplification of the chiral acid via H-bonding is key for the enhancement in reaction rate and yield, while stereocontrol is dependent on the chirality of the acid.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Weil T.; Kotke M.; Kleiner C. M.; Schreiner P. R. Org. Lett. 2008, 10, 1513. 10.1021/ol800149y. - DOI - PubMed
- Zhang Z. G.; Schreiner P. R. Chem. Soc. Rev. 2009, 38, 1187. 10.1039/b801793j. - DOI - PubMed
- Uraguchi D.; Ueki Y.; Ooi T. Science 2009, 326, 120. 10.1126/science.1176758. - DOI - PubMed
- Xu H.; Zuend S. J.; Woll M. G.; Tao Y.; Jacobsen E. N. Science 2010, 327, 986. 10.1126/science.1182826. - DOI - PMC - PubMed
- Hong L.; Sun W. S.; Yang D. X.; Li G. F.; Wang R. Chem. Rev. 2016, 116, 4006. 10.1021/acs.chemrev.5b00676. - DOI - PubMed
-
-
For selected recent examples see:
- Balmond E. I.; Galan M. C.; McGarrigle E. M. Synlett 2013, 24, 2335.and references therein 10.1055/s-0033-1338970. - DOI
- Issa J. P.; Lloyd D.; Steliotes E.; Bennett C. S. Org. Lett. 2013, 15, 4170. 10.1021/ol4018547. - DOI - PubMed
- Zhu D.; Baryal K. N.; Adhikari S.; Zhu J. J. Am. Chem. Soc. 2014, 136, 3172. 10.1021/ja4116956. - DOI - PubMed
- Kaneko M.; Herzon S. B. Org. Lett. 2014, 16, 2776. 10.1021/ol501101f. - DOI - PMC - PubMed
- Chen J. H.; Ruei J. H.; Mong K. K. T. Eur. J. Org. Chem. 2014, 2014, 1827. 10.1002/ejoc.201400006. - DOI
- Issa J. P.; Bennett C. S. J. Am. Chem. Soc. 2014, 136, 5740. 10.1021/ja500410c. - DOI - PubMed
- Wang H.; Tao J. Y.; Cai X. P.; Chen W.; Zhao Y. Q.; Xu Y.; Yao W.; Zeng J.; Wan Q. Chem. - Eur. J. 2014, 20, 17319. 10.1002/chem.201405516. - DOI - PubMed
- Das S.; Pekel D.; Neudorfl J. M.; Berkessel A. Angew. Chem., Int. Ed. 2015, 54, 12479. 10.1002/anie.201503156. - DOI - PubMed
- Medina S.; Galan M. C.. Carbohydrate Chemistry; Royal Society of Chemistry: Cambridge, U.K., 2016; Vol. 41, p 59
- Hsu M. Y.; Liu Y. P.; Lam S.; Lin S. C.; Wang C. C. Beilstein J. Org. Chem. 2016, 12, 1758. 10.3762/bjoc.12.164. - DOI - PMC - PubMed
- Nogueira J. M.; Bylsma M.; Bright D. K.; Bennett C. S. Angew. Chem., Int. Ed. 2016, 55, 10088. 10.1002/anie.201605091. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources