Functional evolution of a morphogenetic gradient
- PMID: 28005004
- PMCID: PMC5224919
- DOI: 10.7554/eLife.20894
Functional evolution of a morphogenetic gradient
Abstract
Bone Morphogenetic Proteins (BMPs) pattern the dorsal-ventral axis of bilaterian embryos; however, their roles in the evolution of body plan are largely unknown. We examined their functional evolution in fly embryos. BMP signaling specifies two extraembryonic tissues, the serosa and amnion, in basal-branching flies such as Megaselia abdita, but only one, the amnioserosa, in Drosophila melanogaster. The BMP signaling dynamics are similar in both species until the beginning of gastrulation, when BMP signaling broadens and intensifies at the edge of the germ rudiment in Megaselia, while remaining static in Drosophila. Here we show that the differences in gradient dynamics and tissue specification result from evolutionary changes in the gene regulatory network that controls the activity of a positive feedback circuit on BMP signaling, involving the tumor necrosis factor alpha homolog eiger. These data illustrate an evolutionary mechanism by which spatiotemporal changes in morphogen gradients can guide tissue complexity.
Keywords: Bone Morphogenetic Protein; D. melanogaster; Megaselia abdita; amnion; developmental biology; eiger; positive feedback; serosa; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




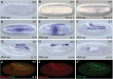

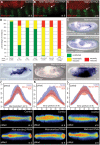






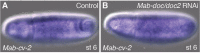
Similar articles
-
BMP-dependent serosa and amnion specification in the scuttle fly Megaselia abdita.Development. 2012 Sep;139(18):3373-82. doi: 10.1242/dev.083873. Epub 2012 Aug 8. Development. 2012. PMID: 22874914
-
BMP signaling components in embryonic transcriptomes of the hover fly Episyrphus balteatus (Syrphidae).BMC Genomics. 2011 May 31;12:278. doi: 10.1186/1471-2164-12-278. BMC Genomics. 2011. PMID: 21627820 Free PMC article.
-
Two consecutive microtubule-based epithelial seaming events mediate dorsal closure in the scuttle fly Megaselia abdita.Elife. 2018 Mar 14;7:e33807. doi: 10.7554/eLife.33807. Elife. 2018. PMID: 29537962 Free PMC article.
-
How two extraembryonic epithelia became one: serosa and amnion features and functions of Drosophila's amnioserosa.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210265. doi: 10.1098/rstb.2021.0265. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252222 Free PMC article. Review.
-
Diversity and robustness of bone morphogenetic protein pattern formation.Development. 2021 Apr 6;148(7):dev192344. doi: 10.1242/dev.192344. Print 2021 Apr 1. Development. 2021. PMID: 33795238 Free PMC article. Review.
Cited by
-
Decoupling from yolk sac is required for extraembryonic tissue spreading in the scuttle fly Megaselia abdita.Elife. 2018 Oct 30;7:e34616. doi: 10.7554/eLife.34616. Elife. 2018. PMID: 30375972 Free PMC article.
-
Extraembryonic tissue in chelicerates: a review and outlook.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210269. doi: 10.1098/rstb.2021.0269. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252223 Free PMC article. Review.
-
The dorsoventral patterning of Musca domestica embryos: insights into BMP/Dpp evolution from the base of the lower cyclorraphan flies.Evodevo. 2018 May 16;9:13. doi: 10.1186/s13227-018-0102-5. eCollection 2018. Evodevo. 2018. PMID: 29796243 Free PMC article.
-
BMPs direct sensory interneuron identity in the developing spinal cord using signal-specific not morphogenic activities.Elife. 2017 Sep 19;6:e30647. doi: 10.7554/eLife.30647. Elife. 2017. PMID: 28925352 Free PMC article.
-
Genomic Resources for the Scuttle Fly Megaselia abdita: A Model Organism for Comparative Developmental Studies in Flies.bioRxiv [Preprint]. 2025 Feb 4:2025.01.13.631075. doi: 10.1101/2025.01.13.631075. bioRxiv. 2025. PMID: 39868096 Free PMC article. Preprint.
References
-
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

