HIV-1 Glycoprotein 120 Enhancement of N-Methyl-D-Aspartate NMDA Receptor-Mediated Excitatory Postsynaptic Currents: Implications for HIV-1-Associated Neural Injury
- PMID: 28005232
- PMCID: PMC5944862
- DOI: 10.1007/s11481-016-9719-0
HIV-1 Glycoprotein 120 Enhancement of N-Methyl-D-Aspartate NMDA Receptor-Mediated Excitatory Postsynaptic Currents: Implications for HIV-1-Associated Neural Injury
Abstract
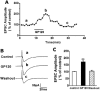
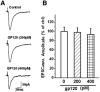
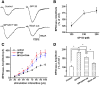
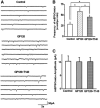
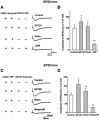
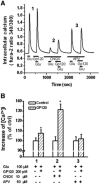
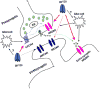
It is widely accepted that human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120) plays an important role in HIV-1-induced neural injury and pathogenesis of HIV-1-associated dementia (HAND). Multiple pathways have been proposed for gp120-induced neurotoxicity, amongst is the activation of N-Methyl-D-Aspartate receptors (NMDARs). It has been shown that gp120 causes neuronal injury or death and gp120 transgenic mice exhibit neurological similarity to that of HAND, all of which can be blocked or attenuated by NMDAR antagonists. Several lines of evidence indicate the subtype and location of activated NMDARs are key determinants of the nature of NMDAR physiology. To examine the subtype and the location of NMDARs affected by gp120, we studied gp120 on subtype NMDAR-mediated EPSCs in the CA1 region of rat hippocampal slices through "blind" whole-cell patch recordings. Our results showed bath application of gp120 increased both NR2A- and NR2B-mediated EPSCs possibly via a presynaptic mechanism, with much stronger effect on NR2B-mediated EPSCs. In contrast, gp120 failed on enhancing AMPA receptor-mediated EPSCs. Ca2+ imaging studies revealed that gp120 potentiated glutamate-induced increase of intracellular Ca2+ concentration in rat hippocampal neuronal cultures which were blocked by a NMDAR antagonist, but not by an AMPA receptor antagonist, indicating gp120 induces Ca2+ influx through NMDARs. Further investigations demonstrated that gp120 increased the EPSCs mediated by extrasynaptic NR2BRs. Taken together, these results demonstrate that gp120 interacts with both NR2A and NR2B subtypes of NMDARs with a predominant action on the extrasynaptic NR2B, implicating a role NR2B may play in HIV-1-associated neuropathology.
Keywords: CA1; Glutamate receptors; HIV; Neurodegeneration; Synaptic transmission.
Figures


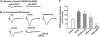
References
-
- Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I. Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotox Res. 2004;5:605–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

