Collagen V expression is crucial in regional development of the supraspinatus tendon
- PMID: 28005290
- PMCID: PMC5189919
- DOI: 10.1002/jor.23246
Collagen V expression is crucial in regional development of the supraspinatus tendon
Abstract
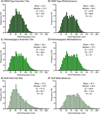
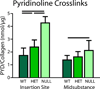
Manipulations in cell culture and mouse models have demonstrated that reduction of collagen V results in altered fibril structure and matrix assembly. A tissue-dependent role for collagen V in determining mechanical function was recently established, but its role in determining regional properties has not been addressed. The objective of this study was to define the role(s) of collagen V expression in establishing the site-specific properties of the supraspinatus tendon. The insertion and midsubstance of tendons from wild type, heterozygous and tendon/ligament-specific null mice were assessed for crimp morphology, fibril morphology, cell morphology, as well as total collagen and pyridinoline cross-link (PYD) content. Fibril morphology was altered at the midsubstance of both groups with larger, but fewer, fibrils and no change in cell morphology or collagen compared to the wild type controls. In contrast, a significant disruption of fibril assembly was observed at the insertion site of the null group with the presence of structurally aberrant fibrils. Alterations were also present in cell density and PYD content. Altogether, these results demonstrate that collagen V plays a crucial role in determining region-specific differences in mouse supraspinatus tendon structure. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:2154-2161, 2016.
Keywords: EDS; collagen; fibril; supraspinatus; tendon.
© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


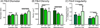
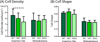

References
-
- Ainsworth SR, Aulicino PL. A survey of patients with Ehlers-Danlos syndrome. Clin Orthop Relat Res. 1993:250–256. - PubMed
-
- Stanitski DF, Nadjarian R, Stanitski CL, et al. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin Orthop Relat Res. 2000:213–221. - PubMed
-
- Symoens S, Syx D, Malfait F, et al. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat. 2012;33:1485–1493. - PubMed
-
- Malfait F, De Paepe A. The Ehlers-Danlos syndrome. Advances in experimental medicine and biology. 2014;802:129–143. - PubMed
-
- Malfait F, Coucke P, Symoens S, et al. The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat. 2005;25:28–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

