The Lr34 adult plant rust resistance gene provides seedling resistance in durum wheat without senescence
- PMID: 28005310
- PMCID: PMC5466443
- DOI: 10.1111/pbi.12684
The Lr34 adult plant rust resistance gene provides seedling resistance in durum wheat without senescence
Abstract
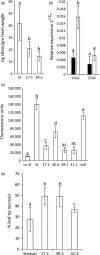
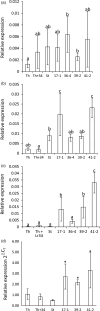
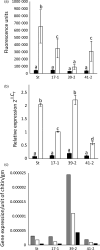
The hexaploid wheat (Triticum aestivum) adult plant resistance gene, Lr34/Yr18/Sr57/Pm38/Ltn1, provides broad-spectrum resistance to wheat leaf rust (Lr34), stripe rust (Yr18), stem rust (Sr57) and powdery mildew (Pm38) pathogens, and has remained effective in wheat crops for many decades. The partial resistance provided by this gene is only apparent in adult plants and not effective in field-grown seedlings. Lr34 also causes leaf tip necrosis (Ltn1) in mature adult plant leaves when grown under field conditions. This D genome-encoded bread wheat gene was transferred to tetraploid durum wheat (T. turgidum) cultivar Stewart by transformation. Transgenic durum lines were produced with elevated gene expression levels when compared with the endogenous hexaploid gene. Unlike nontransgenic hexaploid and durum control lines, these transgenic plants showed robust seedling resistance to pathogens causing wheat leaf rust, stripe rust and powdery mildew disease. The effectiveness of seedling resistance against each pathogen correlated with the level of transgene expression. No evidence of accelerated leaf necrosis or up-regulation of senescence gene markers was apparent in these seedlings, suggesting senescence is not required for Lr34 resistance, although leaf tip necrosis occurred in mature plant flag leaves. Several abiotic stress-response genes were up-regulated in these seedlings in the absence of rust infection as previously observed in adult plant flag leaves of hexaploid wheat. Increasing day length significantly increased Lr34 seedling resistance. These data demonstrate that expression of a highly durable, broad-spectrum adult plant resistance gene can be modified to provide seedling resistance in durum wheat.
Keywords: Blumeria; Puccinia; Triticum; ABC transporter; rust.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Introgression of the bread wheat D genome encoded Lr34/Yr18/Sr57/Pm38/Ltn1 adult plant resistance gene into Triticum turgidum (durum wheat).Theor Appl Genet. 2023 Oct 17;136(11):226. doi: 10.1007/s00122-023-04466-z. Theor Appl Genet. 2023. PMID: 37847385 Free PMC article.
-
The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice.Plant Biotechnol J. 2016 May;14(5):1261-8. doi: 10.1111/pbi.12491. Epub 2015 Oct 15. Plant Biotechnol J. 2016. PMID: 26471973 Free PMC article.
-
Functional variability of the Lr34 durable resistance gene in transgenic wheat.Plant Biotechnol J. 2012 May;10(4):477-87. doi: 10.1111/j.1467-7652.2012.00683.x. Epub 2012 Feb 10. Plant Biotechnol J. 2012. PMID: 22321563
-
Quantitative Trait Loci Conferring Leaf Rust Resistance in Hexaploid Wheat.Phytopathology. 2018 Dec;108(12):1344-1354. doi: 10.1094/PHYTO-06-18-0208-RVW. Epub 2018 Nov 2. Phytopathology. 2018. PMID: 30211634 Review.
-
Wheat leaf rust caused by Puccinia triticina.Mol Plant Pathol. 2008 Sep;9(5):563-75. doi: 10.1111/j.1364-3703.2008.00487.x. Mol Plant Pathol. 2008. PMID: 19018988 Free PMC article. Review.
Cited by
-
Breeding for durable resistance against biotrophic fungal pathogens using transgenes from wheat.Mol Breed. 2024 Jan 22;44(2):8. doi: 10.1007/s11032-024-01451-2. eCollection 2024 Feb. Mol Breed. 2024. PMID: 38263979 Free PMC article.
-
Fine-mapping of LrN3B on wheat chromosome arm 3BS, one of the two complementary genes for adult-plant leaf rust resistance.Theor Appl Genet. 2024 Aug 13;137(9):203. doi: 10.1007/s00122-024-04706-w. Theor Appl Genet. 2024. PMID: 39134836
-
Variations in exons 11 and 12 of the multi-pest resistance wheat gene Lr34 are independently additive for leaf rust resistance.Front Plant Sci. 2023 Feb 23;13:1061490. doi: 10.3389/fpls.2022.1061490. eCollection 2022. Front Plant Sci. 2023. PMID: 36910459 Free PMC article.
-
Exploring the sound-modulated delay in tomato ripening through expression analysis of coding and non-coding RNAs.Ann Bot. 2018 Dec 31;122(7):1231-1244. doi: 10.1093/aob/mcy134. Ann Bot. 2018. PMID: 30010774 Free PMC article.
-
The Wheat Lr67 Gene from the Sugar Transport Protein 13 Family Confers Multipathogen Resistance in Barley.Plant Physiol. 2019 Apr;179(4):1285-1297. doi: 10.1104/pp.18.00945. Epub 2018 Oct 10. Plant Physiol. 2019. PMID: 30305371 Free PMC article.
References
-
- Ayliffe, M.A. , Collins, N.C. , Ellis, J.G. and Pryor, A. (2000) The maize rp1 rust resistance gene identifies homologues in barley that have been subjected to diversifying selection. Theor. Appl. Genet. 100, 1144–1154.
-
- Ayliffe, M.A. , Devilla, R. , Mago, R. , White, R. , Talbot, M. , Pryor, A. and Leung, H. (2011) Non‐host resistance of rice to rust pathogens. Mol. Plant Microbe Interact. 24, 1143–1155. - PubMed
-
- Ayliffe, M. , Periyannan, S. , Feechan, A. , Dry, I. , Schumann, U. , Wang, M.‐B. , Pryor, A. et al. (2013) A simple method for comparing fungal biomass in infected plant tissues. Mol. Plant Microbe Interact. 26, 658–667. - PubMed
-
- Ceoloni, C. , Biagetti, M. , Ciaffi, M. , Forte, P. and Pasquini, M. (1996) Wheat chromosome engineering at the 4x level: the potential of different alien gene transfers into durum wheat. Euphytica, 89, 87–97.
-
- Chauhan, H. , Boni, R. , Bucher, R. , Kuhn, B. , Buchmann, G. , Sucher, J. , Selter, L.L. et al. (2015) The wheat resistance gene Lr34 results in constitutive induction of multiple defense pathways in transgenic barley. Plant J. 84, 202–215. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

