SWEPT-SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY REVEALS CHORIOCAPILLARIS ALTERATIONS IN EYES WITH NASCENT GEOGRAPHIC ATROPHY AND DRUSEN-ASSOCIATED GEOGRAPHIC ATROPHY
- PMID: 28005659
- PMCID: PMC5193240
- DOI: 10.1097/IAE.0000000000001287
SWEPT-SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY REVEALS CHORIOCAPILLARIS ALTERATIONS IN EYES WITH NASCENT GEOGRAPHIC ATROPHY AND DRUSEN-ASSOCIATED GEOGRAPHIC ATROPHY
Abstract
Purpose: To investigate choriocapillaris (CC) alteration in patients with nascent geographic atrophy (nGA) and/or drusen-associated geographic atrophy (DAGA) using swept-source optical coherence tomography angiography (OCTA).
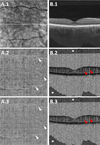
Methods: A 1,050-nm wavelength, 400 kHz A-scan rate swept-source optical coherence tomography prototype was used to perform volumetric swept-source optical coherence tomography angiography over 6 mm × 6 mm fields of view in patients with nGA and/or DAGA. The resulting optical coherence tomography (OCT) and OCTA data were analyzed using a combination of en face and cross-sectional techniques. Variable interscan time analysis (VISTA) was used to differentiate CC flow impairment from complete CC atrophy.
Results: A total of 7 eyes from 6 patients (mean age: 73.8 ± 5.7 years) were scanned. Seven areas of nGA and three areas of DAGA were identified. Analysis of cross-sectional OCT and OCTA images identified focal alterations of the CC underlying all seven areas of nGA and all three areas of DAGA. En face OCTA analysis of the CC revealed diffuse CC alterations in all eyes. Variable interscan time analysis processing suggested that the observed CC flow alterations predominantly corresponded to flow impairment rather than complete CC atrophy.
Conclusion: The OCTA imaging of the CC revealed focal CC flow impairment associated with areas of nGA and DAGA, as well as diffuse CC flow impairment throughout the imaged field. En face OCT analysis should prove useful for understanding the pathogenesis of nGA and DAGA and for identifying the formation of nGA and DAGA as endpoints in therapeutic trials.
Conflict of interest statement
There are no conflicting relationships for any other author.
Figures



References
-
- Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Archives of Ophthalmology. 2009;127:533–540. - PubMed
-
- Sarks JP, Sarks SH, Killingsworth MC. Evolution of Geographic Atrophy of the Retinal-Pigment Epithelium. Eye. 1988;2:552–577. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

