An Intranasal Proteosome-Adjuvanted Trivalent Influenza Vaccine Is Safe, Immunogenic & Efficacious in the Human Viral Influenza Challenge Model. Serum IgG & Mucosal IgA Are Important Correlates of Protection against Illness Associated with Infection
- PMID: 28005959
- PMCID: PMC5179046
- DOI: 10.1371/journal.pone.0163089
An Intranasal Proteosome-Adjuvanted Trivalent Influenza Vaccine Is Safe, Immunogenic & Efficacious in the Human Viral Influenza Challenge Model. Serum IgG & Mucosal IgA Are Important Correlates of Protection against Illness Associated with Infection
Abstract
Introduction: A Proteosome-adjuvanted trivalent inactivated influenza vaccine (P-TIV) administered intra-nasally was shown to be safe, well tolerated and immunogenic in both systemic and mucosal compartments, and effective at preventing illness associated with evidence of influenza infection.
Methods: In two separate studies using the human viral challenge model, subjects were selected to be immunologically naive to A/Panama/2007/1999 (H3N2) virus and then dosed via nasal spray with one of three regimens of P-TIV or placebo. One or two doses, 15 μg or 30 μg, were given either once only or twice 14 days apart (1 x 30 μg, 2 x 30 μg, 2 x 15 μg) and subjects were challenged with A/Panama/2007/1999 (H3N2) virus. Immune responses to the vaccine antigens were measured by haemagglutination inhibition assay (HAI) and nasal wash secretory IgA (sIgA) antibodies.
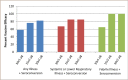
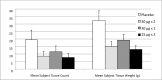
Results: Vaccine reactogenicity was mild, predictable and generally consistent with earlier Phase I studies with this vaccine. Seroconversion to A/Panama/2007/1999 (H3N2), following vaccination but prior to challenge, occurred in 57% to 77% of subjects in active dosing groups and 2% of placebo subjects. The greatest relative rise in sIgA, following vaccination but prior to challenge, was observed in groups that received 2 doses.
Conclusion: Intranasal vaccination significantly protected against influenza (as defined by influenza symptoms combined with A/Panama seroconversion) following challenge with A/Panama/2007/1999 (H3N2). When data were pooled from both studies, efficacy ranged from 58% to 82% in active dosing groups for any influenza symptoms with seroconversion, 67% to 85% for systemic or lower respiratory illness and seroconversion, and 65% to 100% for febrile illness and seroconversion. The two dose regimen was found to be superior to the single dose regimen. In this study, protection against illness associated with evidence of influenza infection (evidence determined by seroconversion) following challenge with virus, significantly correlated with pre-challenge HAI titres (p = 0.0003) and mucosal sIgA (p≤0.0001) individually, and HAI (p = 0.028) and sIgA (p = 0.0014) together. HAI and sIgA levels were inversely related to rates of illness.
Trial registration: ClinicalTrials.gov NCT02522754.
Conflict of interest statement
Competing Interests: This study was partly funded by hVIVO Group PLC., ID Biomedical Corporation of Québec, Analytical Solutions Group, Inc. and ID Biomedical Corporation of Maryland, the respective employers of: Rob Lambkin-Williams, Anthony S. Gilbert, Alex Mann, Richard Broughton, Corey P. Mallett, David Burt, and David He. Patent details: Proteosome influenza vaccine US 6743900 B2. Abstract - Improved forms of vaccines which comprise proteosomes and protein antigens are described. Vaccines which contain influenza HA as the antigen are used for illustration as to demonstrate efficacy. Improvements in the preparation of the vaccines themselves and the proteosome component are also included. Publication number - US6743900 B2. Publication type - Grant Application number - US 09/788,280. Publication date - Jun 1, 2004. Filing date - Feb 15, 2001. Priority date - Feb 15, 2000. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures

Similar articles
-
Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers.J Virol. 2006 May;80(10):4962-70. doi: 10.1128/JVI.80.10.4962-4970.2006. J Virol. 2006. PMID: 16641287 Free PMC article. Clinical Trial.
-
A nasally administered trivalent inactivated influenza vaccine is well tolerated, stimulates both mucosal and systemic immunity, and potentially protects against influenza illness.Vaccine. 2011 Feb 24;29(10):1921-8. doi: 10.1016/j.vaccine.2010.12.100. Epub 2011 Jan 8. Vaccine. 2011. PMID: 21219987 Clinical Trial.
-
Mucosal (SIgA) and serum (IgG) immunologic responses in young adults following intranasal administration of one or two doses of inactivated, trivalent anti-influenza vaccine.Vaccine. 2004 Jun 30;22(20):2566-77. doi: 10.1016/j.vaccine.2003.12.018. Vaccine. 2004. PMID: 15193382
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults.Drugs. 2011 Aug 20;71(12):1591-622. doi: 10.2165/11206860-000000000-00000. Drugs. 2011. PMID: 21861544 Review.
Cited by
-
Noninvasive vaccination against infectious diseases.Hum Vaccin Immunother. 2018 Jul 3;14(7):1717-1733. doi: 10.1080/21645515.2018.1461296. Epub 2018 May 17. Hum Vaccin Immunother. 2018. PMID: 29624470 Free PMC article. Review.
-
An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery.Pharmaceutics. 2021 Mar 27;13(4):455. doi: 10.3390/pharmaceutics13040455. Pharmaceutics. 2021. PMID: 33801614 Free PMC article. Review.
-
Innovative Mucosal Vaccine Formulations Against Influenza A Virus Infections.Front Immunol. 2019 Jul 17;10:1605. doi: 10.3389/fimmu.2019.01605. eCollection 2019. Front Immunol. 2019. PMID: 31379823 Free PMC article. Review.
-
Adjuvanted influenza vaccines.Hum Vaccin Immunother. 2018 Mar 4;14(3):550-564. doi: 10.1080/21645515.2017.1415684. Epub 2018 Jan 25. Hum Vaccin Immunother. 2018. PMID: 29232151 Free PMC article. Review.
-
Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission.Curr Immunol Rev. 2019;15(1):102-122. doi: 10.2174/1573395514666180605092054. Curr Immunol Rev. 2019. PMID: 31452652 Free PMC article.
References
-
- Calfee DP, Hayden FG (1998) New approaches to influenza chemotherapy. Neuraminidase inhibitors. Drugs 56: 537–553. - PubMed
-
- Calfee DP, Peng AW, Hussey EK, Lobo M, Hayden FG (1999) Safety and efficacy of once daily intranasal zanamivir in preventing experimental human influenza A infection. Antivir Ther 4: 143–149. - PubMed
-
- Barroso L, Treanor J, Gubareva L, Hayden FG (2005) Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir Ther 10: 901–910. - PubMed
-
- Hayden FG, Treanor JJ, Betts RF, Lobo M, Esinhart JD, Hussey EK (1996) Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA 275: 295–299. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

