Detectable Viral Load in Late Pregnancy among Women in the Rwanda Option B+ PMTCT Program: Enrollment Results from the Kabeho Study
- PMID: 28006001
- PMCID: PMC5179044
- DOI: 10.1371/journal.pone.0168671
Detectable Viral Load in Late Pregnancy among Women in the Rwanda Option B+ PMTCT Program: Enrollment Results from the Kabeho Study
Abstract
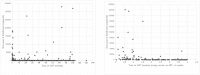
There are limited viral load (VL) data available from programs implementing "Option B+," lifelong antiretroviral treatment (ART) to all HIV-positive pregnant and postpartum women, in resource-limited settings. Extent of viral suppression from a prevention of mother-to-child transmission of HIV program in Rwanda was assessed among women enrolled in the Kigali Antiretroviral and Breastfeeding Assessment for the Elimination of HIV (Kabeho) Study. ARV drug resistance testing was conducted on women with VL>2000 copies/ml. In April 2013-January 2014, 608 pregnant or early postpartum HIV-positive women were enrolled in 14 facilities. Factors associated with detectable enrollment VL (>20 copies/ml) were examined using generalized estimating equations. The most common antiretroviral regimen (56.7%, 344/607) was tenofovir/lamivudine/efavirenz. Median ART duration was 13.5 months (IQR 3.0-48.8); 76.1% of women were on ART at first antenatal visit. Half of women (315/603) had undetectable RNA-PCR VL and 84.6% (510) had <1,000 copies/ml. Detectable VL increased among those on ART > 36 months compared to those on ART 4-36 months (72/191, 37.7% versus 56/187, 29.9%), though the difference was not significant. The odds of having detectable enrollment VL decreased significantly as duration on ART at enrollment increased (AOR = 0.99, 95% CI: 0.9857, 0.9998, p = 0.043). There was a higher likelihood of detectable VL for women with lower gravidity (AOR = 0.90, 95% CI: 0.84, 0.97, p = 0.0039), no education (AOR = 2.25, (95% CI: 1.37, 3.70, p = 0.0004), nondisclosure to partner (AOR = 1.97, 95% CI: 1.21, 3.21, p = 0.0063) and side effects (AOR = 2.63, 95% CI: 1.72, 4.03, p<0.0001). ARV drug resistance mutations were detected in all of the eleven women on ART > 36 months with genotyping available. Most women were receiving ART at first antenatal visit, with relatively high viral suppression rates. Shorter ART duration was associated with higher VL, with a concerning increasing trend for higher viremia and drug resistance among women on ART for >3 years.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO Department of HIV/AIDS;2013. - PubMed
-
- Rwanda Biomedical Center. National Guidelines for Prevention and Management of HIV, STIs & Other Blood Borne Infections. Kigali, Rwanda: Ministry of Health, Republic of Rwanda; 2013.
-
- Hoffman RM, Black V, Technau K, van der Merwe KJ, Currier J, Coovadia A, et al. Effects of Highly Active Antiretroviral Therapy Duration and Regimen on Risk for Mother-to-Child Transmission of HIV in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2010;54: 35–41. 10.1097/QAI.0b013e3181cf9979 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

