Selection and Characterization of Tau Binding ᴅ-Enantiomeric Peptides with Potential for Therapy of Alzheimer Disease
- PMID: 28006031
- PMCID: PMC5179029
- DOI: 10.1371/journal.pone.0167432
Selection and Characterization of Tau Binding ᴅ-Enantiomeric Peptides with Potential for Therapy of Alzheimer Disease
Abstract
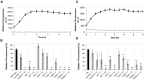
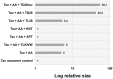
A variety of neurodegenerative disorders, including Alzheimer disease (AD), are associated with neurofibrillary tangles composed of the tau protein, as well as toxic tau oligomers. Inhibitors of pathological tau aggregation, interrupting tau self-assembly, might be useful for the development of therapeutics. Employing mirror image phage display with a large peptide library (over 109 different peptides), we have identified tau fibril binding peptides consisting of d-enantiomeric amino acids. d-enantiomeric peptides are extremely protease stable and not or less immunogenic than l-peptides, and the suitability of d-peptides for in vivo applications have already been demonstrated. Phage display selections were performed using fibrils of the d-enantiomeric hexapeptide VQIVYK, representing residues 306 to 311 of the tau protein, as a target. VQIVYK has been demonstrated to be important for fibril formation of the full lengths protein and forms fibrils by itself. Here, we report on d-enantiomeric peptides, which bind to VQIVYK, tau isoforms like tau3RD (K19) as well as to full lengths tau fibrils, and modulate the aggregation of the respective tau form. The peptides are able to penetrate cells and might be interesting for therapeutic and diagnostic applications in AD research.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Potent Tau Aggregation Inhibitor D-Peptides Selected against Tau-Repeat 2 Using Mirror Image Phage Display.Chembiochem. 2021 Nov 3;22(21):3049-3059. doi: 10.1002/cbic.202100287. Epub 2021 Sep 12. Chembiochem. 2021. PMID: 34375027 Free PMC article.
-
Mirror-Image Phage Display for the Selection of D-Amino Acid Peptide Ligands as Potential Therapeutics.Curr Protoc. 2024 Feb;4(2):e957. doi: 10.1002/cpz1.957. Curr Protoc. 2024. PMID: 38372457
-
Selection of a d-Enantiomeric Peptide Specifically Binding to PHF6 for Inhibiting Tau Aggregation in Transgenic Mice.ACS Chem Neurosci. 2020 Dec 16;11(24):4240-4253. doi: 10.1021/acschemneuro.0c00518. Epub 2020 Dec 7. ACS Chem Neurosci. 2020. PMID: 33284003
-
Probable participation of 14-3-3 in tau protein oligomerization and aggregation.J Alzheimers Dis. 2011;27(3):467-76. doi: 10.3233/JAD-2011-110692. J Alzheimers Dis. 2011. PMID: 21876254 Review.
-
Tauopathies and tau oligomers.J Alzheimers Dis. 2013;37(3):565-8. doi: 10.3233/JAD-130653. J Alzheimers Dis. 2013. PMID: 23948895 Review.
Cited by
-
Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer's disease-like symptoms in animal model.Cell Mol Life Sci. 2020 Jul;77(14):2795-2813. doi: 10.1007/s00018-019-03312-0. Epub 2019 Sep 27. Cell Mol Life Sci. 2020. PMID: 31562564 Free PMC article.
-
Nanoplatforms Targeting Intrinsically Disordered Protein Aggregation for Translational Neuroscience Applications.Nanomaterials (Basel). 2025 May 8;15(10):704. doi: 10.3390/nano15100704. Nanomaterials (Basel). 2025. PMID: 40423094 Free PMC article. Review.
-
Phage Display Technology in Biomarker Identification with Emphasis on Non-Cancerous Diseases.Molecules. 2024 Jun 25;29(13):3002. doi: 10.3390/molecules29133002. Molecules. 2024. PMID: 38998954 Free PMC article. Review.
-
N-Amination Converts Amyloidogenic Tau Peptides into Soluble Antagonists of Cellular Seeding.ACS Chem Neurosci. 2021 Oct 20;12(20):3928-3938. doi: 10.1021/acschemneuro.1c00528. Epub 2021 Oct 5. ACS Chem Neurosci. 2021. PMID: 34609825 Free PMC article.
-
D-Peptide and D-Protein Technology: Recent Advances, Challenges, and Opportunities.Chembiochem. 2023 Feb 14;24(4):e202200537. doi: 10.1002/cbic.202200537. Epub 2022 Nov 16. Chembiochem. 2023. PMID: 36278392 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

