Association of DNA Mismatch Repair and Mutations in BRAF and KRAS With Survival After Recurrence in Stage III Colon Cancers : A Secondary Analysis of 2 Randomized Clinical Trials
- PMID: 28006055
- PMCID: PMC5498991
- DOI: 10.1001/jamaoncol.2016.5469
Association of DNA Mismatch Repair and Mutations in BRAF and KRAS With Survival After Recurrence in Stage III Colon Cancers : A Secondary Analysis of 2 Randomized Clinical Trials
Abstract
Importance: The association of biomarkers with patient survival after recurrence (SAR) of cancer is poorly understood but may guide management and treatment.
Objective: To determine the association of DNA mismatch repair (MMR) status and somatic mutation in the B-Raf proto-oncogene (c.1799T>A [V600E]; BRAFV600E) or exon 2 of the KRAS proto-oncogene (KRAS) in the primary tumor with SAR in patients with stage III colon carcinomas treated with adjuvant chemotherapy.
Design, setting, and participants: Patients with resected stage III colon cancers were randomized to adjuvant FOLFOX (folinic acid [leucovorin calcium], fluorouracil, and oxaliplatin) chemotherapy with or without cetuximab (North Central Cancer Treatment Group N0147 trial) or adjuvant FOLFOX chemotherapy with or without bevacizumab (National Surgical Adjuvant Breast and Bowel Project C-08 trial). Associations of biomarkers with SAR were analyzed using Cox proportional hazards models adjusted for clinicopathologic features and time to recurrence (data collected February 10, 2004, to August 7, 2015).
Main outcomes and measures: The primary study outcome was survival after recurrence of cancer. A secondary outcome measure was the effect of the site of the primary tumor on the association of biomarkers with SAR.
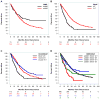
Results: Among 871 patients with cancer recurrence in the N0147 trial (472 men [54.2%] and 399 women [45.8%]; mean [SD] age, 57.8 [11.2] years) and 524 in the C-08 trial (269 men [51.3%] and 255 women [48.7%]; mean [SD] age, 57.0 [11.7] years), multivariable analysis revealed that patients whose tumors had deficient vs proficient MMR had significantly better SAR (adjusted hazard ratio [AHR], 0.70; 95% CI, 0.52-0.96; P = .03). Patients whose tumors harbored mutant BRAFV600E (AHR, 2.45; 95% CI, 1.85-3.25; P < .001) or mutant KRAS (AHR, 1.21; 95% CI, 1.00-1.47; P = .052) had worse SAR compared with those whose tumors had wild-type copies of both genes, although only results for BRAFV600E achieved statistical significance. Significant interactions were found for MMR (P = .03) and KRAS (P = .02) by primary tumor site for SAR. Improved SAR was observed for patients with deficient MMR tumors of the proximal vs distal colon (AHR, 0.57; 95% CI, 0.40-0.83; P = .003), and worse SAR was observed for tumors of the distal colon with mutant KRAS in codon 12 (AHR, 1.76; 95% CI, 1.30-2.38; P < .001) and codon 13 (AHR, 1.76; 95% CI, 1.08-2.86; P = .02).
Conclusions and relevance: In patients with recurrence of stage III colon cancer, deficient MMR was significantly associated with better SAR, and this benefit was limited to primary tumors of the proximal colon. Mutations in BRAFV600E were significantly associated with worse SAR, and worse SAR for BRAFV600E or KRAS mutant tumors was more strongly associated with distal cancers. These biomarkers have implications for patient management at recurrence.
Trial registration: clinicaltrials.gov Identifiers: NCT00079274 and NCT00096278.
Conflict of interest statement
The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest with the content of this manuscript.
Figures

Comment in
-
Molecular Markers Beyond Microsatellite Instability for Assessing Prognosis in Early-Stage Colorectal Cancer: What Happens at Relapse?JAMA Oncol. 2017 Apr 1;3(4):481-482. doi: 10.1001/jamaoncol.2016.5463. JAMA Oncol. 2017. PMID: 28006040 No abstract available.
-
Mutation in BRAF V600E: A Poor Prognostic Marker in Stage III Colon Cancers With Deficient MMR?JAMA Oncol. 2017 Sep 1;3(9):1284-1285. doi: 10.1001/jamaoncol.2017.0262. JAMA Oncol. 2017. PMID: 28617898 No abstract available.
-
Mutation in BRAF V600E-Reply.JAMA Oncol. 2017 Sep 1;3(9):1285-1286. doi: 10.1001/jamaoncol.2017.1474. JAMA Oncol. 2017. PMID: 28617912 No abstract available.
References
-
- Klingbiel D, Saridaki Z, Roth AD, Bosman FT, Delorenzi M, Tejpar S. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol. 2015 Jan;26(1):126–132. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U24 CA114740/CA/NCI NIH HHS/United States
- R01 CA210509/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- U24 CA196067/CA/NCI NIH HHS/United States
- K05 CA142885/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

