Effects of Antifouling Biocides on Molecular and Biochemical Defense System in the Gill of the Pacific Oyster Crassostrea gigas
- PMID: 28006823
- PMCID: PMC5179263
- DOI: 10.1371/journal.pone.0168978
Effects of Antifouling Biocides on Molecular and Biochemical Defense System in the Gill of the Pacific Oyster Crassostrea gigas
Abstract
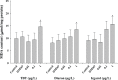
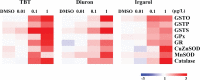
Antifouling biocides such as organotin compounds and their alternatives are potent toxicants in marine ecosystems. In this study, we employed several molecular and biochemical response systems of the Pacific oyster Crassostrea gigas to understand a potential mode of action of antifouling biocides (i.e. tributyltin (TBT), diuron and irgarol) after exposure to different concentrations (0.01, 0.1, and 1 μg L-1) for 96 h. As a result, all the three antifouling biocides strongly induced the antioxidant defense system. TBT reduced both enzymatic activity and mRNA expression of Na+/K+-ATPase and acetylcholinesterase (AChE). Lower levels of both Na+/K+-ATPase activity and AChE mRNA expression were observed in the diuron-exposed oysters compared to the control, while the irgarol treatment reduced only the transcriptional expression of AChE gene. We also analyzed transcript profile of heat shock protein (Hsp) superfamily in same experimental conditions. All antifouling biocides tested in this study significantly modulated mRNA expression of Hsp superfamily with strong induction of Hsp70 family. Taken together, overall results indicate that representative organotin TBT and alternatives have potential hazardous effects on the gill of C. gigas within relatively short time period. Our results also suggest that analyzing a series of molecular and biochemical parameters can be a way of understanding and uncovering the mode of action of emerging antifouling biocides. In particular, it was revealed that Pacific oysters have different sensitivities depend on the antifouling biocides.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Lewis JA, editor Marine biofouling and its prevention on underwater surfaces. Materials Forum; 1998.
-
- Alzieu CL, Sanjuan J, Deltreil JP, Borel M. Tin contamination in Arcachon Bay: Effects on oyster shell anomalies. Mar Pollut Bull. 1986;17(11):494–8.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

