Study protocol of the Asian XELIRI ProjecT (AXEPT): a multinational, randomized, non-inferiority, phase III trial of second-line chemotherapy for metastatic colorectal cancer, comparing the efficacy and safety of XELIRI with or without bevacizumab versus FOLFIRI with or without bevacizumab
- PMID: 28007025
- PMCID: PMC5178089
- DOI: 10.1186/s40880-016-0166-3
Study protocol of the Asian XELIRI ProjecT (AXEPT): a multinational, randomized, non-inferiority, phase III trial of second-line chemotherapy for metastatic colorectal cancer, comparing the efficacy and safety of XELIRI with or without bevacizumab versus FOLFIRI with or without bevacizumab
Abstract
Background: Capecitabine and irinotecan combination therapy (XELIRI) has been examined at various dose levels to treat metastatic colorectal cancer (mCRC). Recently, in the Association of Medical Oncology of the German Cancer Society (AIO) 0604 trial, tri-weekly XELIRI plus bevacizumab, with reduced doses of irinotecan (200 mg/m2 on day 1) and capecitabine (1600 mg/m2 on days 1-14), repeated every 3 weeks, has shown favorable tolerability and efficacy which were comparable to those of capecitabine and oxaliplatin (XELOX) plus bevacizumab. The doses of capecitabine and irinotecan in the AIO trial are considered optimal. In a phase I/II study, XELIRI plus bevacizumab (BIX) as second-line chemotherapy was well tolerated and had promising efficacy in Japanese patients.
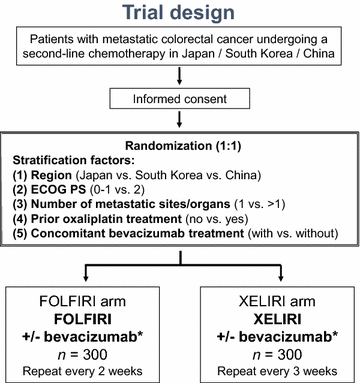
Methods: The Asian XELIRI ProjecT (AXEPT) is an East Asian collaborative, open-labelled, randomized, phase III clinical trial which was designed to demonstrate the non-inferiority of XELIRI with or without bevacizumab versus standard FOLFIRI (5-fluorouracil, leucovorin, and irinotecan combination) with or without bevacizumab as second-line chemotherapy for patients with mCRC. Patients with 20 years of age or older, histologically confirmed mCRC, Eastern Cooperative Oncology Group performance status 0-2, adequate organ function, and disease progression or intolerance of the first-line regimen will be eligible. Patients will be randomized (1:1) to receive standard FOLFIRI with or without bevacizumab (5 mg/kg on day 1), repeated every 2 weeks (FOLIRI arm) or XELIRI with or without bevacizumab (7.5 mg/kg on day 1), repeated every 3 weeks (XELIRI arm). A total of 464 events were estimated as necessary to show non-inferiority with a power of 80% at a one-sided α of 0.025, requiring a target sample size of 600 patients. The 95% confidence interval (CI) upper limit of the hazard ratio was pre-specified as less than 1.3.
Conclusion: The Asian XELIRI ProjecT is a multinational phase III trial being conducted to provide evidence for XELIRI with or without bevacizumab as a second-line treatment option of mCRC. Trial registration ClinicalTrials.gov NCT01996306. UMIN000012263.
Keywords: Bevacizumab; Metastatic colorectal cancer; Randomized phase III clinical trial; Second-line therapy; XELIRI.
Figures
References
-
- National comprehensive cancer network clinical practice guidelines in oncology colon cancer Version 3. 2015. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 6 Aug 2015.
-
- Japanese society for cancer of the colon and rectum: JSCCR Guidelines 2014 for the treatment of colorectal cancer. Tokyo: Kanehara & Co., Ltd; 2010.
-
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;10(15):1065–1075. doi: 10.1016/S1470-2045(14)70330-4. - DOI - PubMed
-
- Venook AP, Niedzwiecki D, Lenz H, Innocenti F, Mahoney MR, O’Neil BH, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;32(15):3. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

