Phase I/II trial evaluating concurrent carbon-ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma
- PMID: 28007028
- PMCID: PMC5178073
- DOI: 10.1186/s40880-016-0164-5
Phase I/II trial evaluating concurrent carbon-ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma
Abstract
Background: After definitive chemoradiotherapy for non-metastatic nasopharyngeal carcinoma (NPC), more than 10% of patients will experience a local recurrence. Salvage treatments present significant challenges for locally recurrent NPC. Surgery, stereotactic ablative body radiotherapy, and brachytherapy have been used to treat locally recurrent NPC. However, only patients with small-volume tumors can benefit from these treatments. Re-irradiation with X-ray-based intensity-modulated radiotherapy (IMXT) has been more widely used for salvage treatment of locally recurrent NPC with a large tumor burden, but over-irradiation to the surrounding normal tissues has been shown to cause frequent and severe toxicities. Furthermore, locally recurrent NPC represents a clinical entity that is more radio-resistant than its primary counterpart. Due to the inherent physical advantages of heavy-particle therapy, precise dose delivery to the target volume(s), without exposing the surrounding organs at risk to extra doses, is highly feasible with carbon-ion radiotherapy (CIRT). In addition, CIRT is a high linear energy transfer (LET) radiation and provides an increased relative biological effectiveness compared with photon and proton radiotherapy. Our prior work showed that CIRT alone to 57.5 GyE (gray equivalent), at 2.5 GyE per daily fraction, was well tolerated in patients who were previously treated for NPC with a definitive dose of IMXT. The short-term response rates at 3-6 months were also acceptable. However, no patients were treated with concurrent chemotherapy. Whether the addition of concurrent chemotherapy to CIRT can benefit locally recurrent NPC patients over CIRT alone has never been addressed. It is possible that the benefits of high-LET CIRT may make radiosensitizing chemotherapy unnecessary. We therefore implemented a phase I/II clinical trial to address these questions and present our methodology and results.
Methods and design: The maximal tolerated dose (MTD) of re-treatment using raster-scanning CIRT plus concurrent cisplatin will be determined in the phase I, dose-escalating stage of this study. CIRT dose escalation from 52.5 to 65 GyE (2.5 GyE × 21-26 fractions) will be delivered, with the primary endpoints being acute and subacute toxicities. Efficacy in terms of overall survival (OS) and local progression-free survival of patients after concurrent chemotherapy plus CIRT at the determined MTD will then be studied in the phase II stage of the trial. We hypothesize that CIRT plus chemotherapy can improve the 2-year OS rate from the historical 50% to at least 70%.
Conclusions: Re-treatment of locally recurrent NPC using photon radiation techniques, including IMXT, provides moderate efficacy but causes potentially severe toxicities. Improved outcomes in terms of efficacy and toxicity profile are expected with CIRT plus chemotherapy. However, the MTD of CIRT used concurrently with cisplatin-based chemotherapy for locally recurrent NPC remains to be determined. In addition, whether the addition of chemotherapy to CIRT is needed remains unknown. These questions will be evaluated in the dose-escalating phase I and randomized phase II trials.
Keywords: Carbon ion radiotherapy; Chemotherapy; Re-irradiation; Recurrent nasopharyngeal cancer; Salvage therapy.
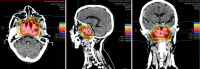
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

